Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells
- PMID: 23349986
- PMCID: PMC3551902
- DOI: 10.1371/journal.pone.0054889
Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells
Abstract
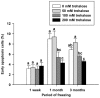
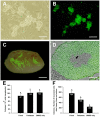
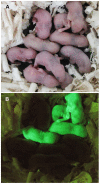
Development of techniques to isolate, culture, and transplant human spermatogonial stem cells (SSCs) has the future potential to treat male infertility. To maximize the efficiency of these techniques, methods for SSC cryopreservation need to be developed to bank SSCs for extended periods of time. Although, it has been demonstrated that SSCs can reinitiate spermatogenesis after freezing, optimal cryopreservation protocols that maximize SSC proliferative capacity post-thaw have not been identified. The objective of this study was to develop an efficient cryopreservation technique for preservation of SSCs. To identify efficient cryopreservation methods for long-term preservation of SSCs, isolated testis cells enriched for SSCs were placed in medium containing dimethyl sulfoxide (DMSO) or DMSO and trehalose (50 mM, 100 mM, or 200 mM), and frozen in liquid nitrogen for 1 week, 1 month, or 3 months. Freezing in 50 mM trehalose resulted in significantly higher cell viability compared to DMSO at all thawing times and a higher proliferation rate compared to DMSO for the 1 week freezing period. Freezing in 200 mM trehalose did not result in increased cell viability; however, proliferation activity was significantly higher and percentage of apoptotic cells was significantly lower compared to DMSO after freezing for 1 and 3 months. To confirm the functionality of SSCs frozen in 200 mM trehalose, SSC transplantation was performed. Donor SSCs formed spermatogenic colonies and sperm capable of generating normal progeny. Collectively, these results indicate that freezing in DMSO with 200 mM trehalose serves as an efficient method for the cryopreservation of SSCs.
Conflict of interest statement
Figures


References
-
- Ewing LL, Davis JC, Zirkin BR (1980) Regulation of testicular function: A spatial and temporal view. Int Rev Physiol 22: 41–115. - PubMed
-
- Russell LD, Ettlin RA, Hikim AP, Clegg ED (1990) Mammalian spermatogenesis. In Histological and Histopathological Evaluation of the Testis. Cache River Press, Clearwater, Florida, USA. 1–40.
-
- De Rooij DG, Van Dissel-Emiliani FMF, Van Pelt AMM (1992) Regulation of spermatogonial proliferation. Annu NY Acad Sci 364: 140–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

