Antigenicity and diagnostic potential of vaccine candidates in human Chagas disease
- PMID: 23350012
- PMCID: PMC3547861
- DOI: 10.1371/journal.pntd.0002018
Antigenicity and diagnostic potential of vaccine candidates in human Chagas disease
Abstract
Background: Chagas disease, caused by Trypanosoma cruzi, is endemic in Latin America and an emerging infectious disease in the US and Europe. We have shown TcG1, TcG2, and TcG4 antigens elicit protective immunity to T. cruzi in mice and dogs. Herein, we investigated antigenicity of the recombinant proteins in humans to determine their potential utility for the development of next generation diagnostics for screening of T. cruzi infection and Chagas disease.
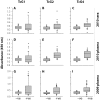
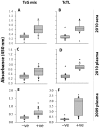
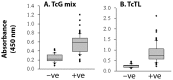
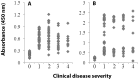
Methods and results: Sera samples from inhabitants of the endemic areas of Argentina-Bolivia and Mexico-Guatemala were analyzed in 1(st)-phase for anti-T. cruzi antibody response by traditional serology tests; and in 2(nd)-phase for antibody response to the recombinant antigens (individually or mixed) by an ELISA. We noted similar antibody response to candidate antigens in sera samples from inhabitants of Argentina and Mexico (n=175). The IgG antibodies to TcG1, TcG2, and TcG4 (individually) and TcG(mix) were present in 62-71%, 65-78% and 72-82%, and 89-93% of the subjects, respectively, identified to be seropositive by traditional serology. Recombinant TcG1- (93.6%), TcG2- (96%), TcG4- (94.6%) and TcG(mix)- (98%) based ELISA exhibited significantly higher specificity compared to that noted for T. cruzi trypomastigote-based ELISA (77.8%) in diagnosing T. cruzi-infection and avoiding cross-reactivity to Leishmania spp. No significant correlation was noted in the sera levels of antibody response and clinical severity of Chagas disease in seropositive subjects.
Conclusions: Three candidate antigens were recognized by antibody response in chagasic patients from two distinct study sites and expressed in diverse strains of the circulating parasites. A multiplex ELISA detecting antibody response to three antigens was highly sensitive and specific in diagnosing T. cruzi infection in humans, suggesting that a diagnostic kit based on TcG1, TcG2 and TcG4 recombinant proteins will be useful in diverse situations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Immunity and vaccine development efforts against Trypanosoma cruzi.Acta Trop. 2019 Dec;200:105168. doi: 10.1016/j.actatropica.2019.105168. Epub 2019 Sep 9. Acta Trop. 2019. PMID: 31513763 Free PMC article. Review.
-
TcG2/TcG4 DNA Vaccine Induces Th1 Immunity Against Acute Trypanosoma cruzi Infection: Adjuvant and Antigenic Effects of Heterologous T. rangeli Booster Immunization.Front Immunol. 2019 Jun 26;10:1456. doi: 10.3389/fimmu.2019.01456. eCollection 2019. Front Immunol. 2019. PMID: 31293599 Free PMC article.
-
Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice.Clin Vaccine Immunol. 2008 Aug;15(8):1158-64. doi: 10.1128/CVI.00144-08. Epub 2008 Jun 11. Clin Vaccine Immunol. 2008. PMID: 18550728 Free PMC article.
-
Delivery of antigenic candidates by a DNA/MVA heterologous approach elicits effector CD8(+)T cell mediated immunity against Trypanosoma cruzi.Vaccine. 2012 Nov 26;30(50):7179-86. doi: 10.1016/j.vaccine.2012.10.018. Epub 2012 Oct 15. Vaccine. 2012. PMID: 23079191 Free PMC article.
-
Diversity of Chagas disease diagnostic antigens: Successes and limitations.PLoS Negl Trop Dis. 2024 Oct 1;18(10):e0012512. doi: 10.1371/journal.pntd.0012512. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39352878 Free PMC article. Review.
Cited by
-
Neglected tropical disease (NTD) diagnostics: current development and operations to advance control.Pathog Glob Health. 2024 Feb;118(1):1-24. doi: 10.1080/20477724.2023.2272095. Epub 2024 Jan 2. Pathog Glob Health. 2024. PMID: 37872790 Free PMC article. Review.
-
Developments in the management of Chagas cardiomyopathy.Expert Rev Cardiovasc Ther. 2015 Dec;13(12):1393-409. doi: 10.1586/14779072.2015.1103648. Epub 2015 Oct 23. Expert Rev Cardiovasc Ther. 2015. PMID: 26496376 Free PMC article. Review.
-
Immunity and vaccine development efforts against Trypanosoma cruzi.Acta Trop. 2019 Dec;200:105168. doi: 10.1016/j.actatropica.2019.105168. Epub 2019 Sep 9. Acta Trop. 2019. PMID: 31513763 Free PMC article. Review.
-
Antigen-Based Nano-Immunotherapy Controls Parasite Persistence, Inflammatory and Oxidative Stress, and Cardiac Fibrosis, the Hallmarks of Chronic Chagas Cardiomyopathy, in A Mouse Model of Trypanosoma cruzi Infection.Vaccines (Basel). 2020 Feb 21;8(1):96. doi: 10.3390/vaccines8010096. Vaccines (Basel). 2020. PMID: 32098116 Free PMC article.
-
TcG2/TcG4 DNA Vaccine Induces Th1 Immunity Against Acute Trypanosoma cruzi Infection: Adjuvant and Antigenic Effects of Heterologous T. rangeli Booster Immunization.Front Immunol. 2019 Jun 26;10:1456. doi: 10.3389/fimmu.2019.01456. eCollection 2019. Front Immunol. 2019. PMID: 31293599 Free PMC article.
References
-
- Schmunis GA, Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115: 14–21. - PubMed
-
- Young C, Losikoff P, Chawla A, Glasser L, Forman E (2007) Transfusion-acquired Trypanosoma cruzi infection. Transfusion 47: 540–544. - PubMed
-
- Munoz J, Portus M, Corachan M, Fumado V, Gascon J (2007) Congenital Trypanosoma cruzi infection in a non-endemic area. Trans R Soc Trop Med Hyg 101: 1161–1162. - PubMed
-
- Rassi A Jr, Rassi A, Marin-Neto JA (2011) Chagas disease. Lancet 375: 1388–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

