Beating the heat--fast scanning melts silk beta sheet crystals
- PMID: 23350037
- PMCID: PMC3553460
- DOI: 10.1038/srep01130
Beating the heat--fast scanning melts silk beta sheet crystals
Abstract
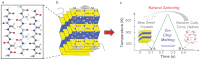
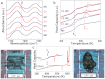
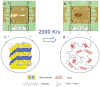
Beta-pleated-sheet crystals are among the most stable of protein secondary structures, and are responsible for the remarkable physical properties of many fibrous proteins, such as silk, or proteins forming plaques as in Alzheimer's disease. Previous thinking, and the accepted paradigm, was that beta-pleated-sheet crystals in the dry solid state were so stable they would not melt upon input of heat energy alone. Here we overturn that assumption and demonstrate that beta-pleated-sheet crystals melt directly from the solid state to become random coils, helices, and turns. We use fast scanning chip calorimetry at 2,000 K/s and report the first reversible thermal melting of protein beta-pleated-sheet crystals, exemplified by silk fibroin. The similarity between thermal melting behavior of lamellar crystals of synthetic polymers and beta-pleated-sheet crystals is confirmed. Significance for controlling beta-pleated-sheet content during thermal processing of biomaterials, as well as towards disease therapies, is envisioned based on these new findings.
Figures

Similar articles
-
Silk I and Silk II studied by fast scanning calorimetry.Acta Biomater. 2017 Jun;55:323-332. doi: 10.1016/j.actbio.2017.04.001. Epub 2017 Apr 5. Acta Biomater. 2017. PMID: 28389368
-
Mechanism of enzymatic degradation of beta-sheet crystals.Biomaterials. 2010 Apr;31(10):2926-33. doi: 10.1016/j.biomaterials.2009.12.026. Epub 2009 Dec 30. Biomaterials. 2010. PMID: 20044136 Free PMC article.
-
Carbonization of a stable β-sheet-rich silk protein into a pseudographitic pyroprotein.Nat Commun. 2015 May 20;6:7145. doi: 10.1038/ncomms8145. Nat Commun. 2015. PMID: 25990218 Free PMC article.
-
Rippled Sheets: The Early Polyglycine Days and Recent Developments in Nylons.Chembiochem. 2022 Mar 4;23(5):e202100658. doi: 10.1002/cbic.202100658. Epub 2022 Feb 2. Chembiochem. 2022. PMID: 35107198 Review.
-
Structure of Silk I (Bombyx mori Silk Fibroin before Spinning) -Type II β-Turn, Not α-Helix.Molecules. 2021 Jun 17;26(12):3706. doi: 10.3390/molecules26123706. Molecules. 2021. PMID: 34204550 Free PMC article. Review.
Cited by
-
The Application of Regenerated Silk Fibroin in Tissue Repair.Materials (Basel). 2024 Aug 7;17(16):3924. doi: 10.3390/ma17163924. Materials (Basel). 2024. PMID: 39203101 Free PMC article. Review.
-
Silk patterns made by direct femtosecond laser writing.Biomicrofluidics. 2016 Sep 2;10(5):054101. doi: 10.1063/1.4962294. eCollection 2016 Sep. Biomicrofluidics. 2016. PMID: 27679677 Free PMC article.
-
Recent Advances in 3D Printing with Protein-Based Inks.Prog Polym Sci. 2021 Apr;115:101375. doi: 10.1016/j.progpolymsci.2021.101375. Epub 2021 Feb 16. Prog Polym Sci. 2021. PMID: 33776158 Free PMC article.
-
Experimental Methods for Characterizing the Secondary Structure and Thermal Properties of Silk Proteins.Macromol Rapid Commun. 2019 Jan;40(1):e1800390. doi: 10.1002/marc.201800390. Epub 2018 Aug 2. Macromol Rapid Commun. 2019. PMID: 30073740 Free PMC article. Review.
-
Emerging Silk Material Trends: Repurposing, Phase Separation and Solution-Based Designs.Materials (Basel). 2021 Mar 1;14(5):1160. doi: 10.3390/ma14051160. Materials (Basel). 2021. PMID: 33804578 Free PMC article. Review.
References
-
- Shao Z. & Vollrath F. Materials: Surprising strength of silkworm silk. Nature 418, 741–741 (2002). - PubMed
-
- Lee S.-M. et al. Greatly increased toughness of infiltrated spider silk. Science 324, 488–492 (2009). - PubMed
-
- Holland C., Vollrath F., Ryan A. J. & Mykhaylyk O. O. Silk and synthetic polymers: reconciling 100 degrees of separation. Adv. Mater. 24, 105–109 (2012). - PubMed
-
- Altman G. H. et al. Silk-based biomaterials. Biomater. 24, 401–416 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

