Molecular and particulate depositions for regional myocardial flows in sheep
- PMID: 2335030
- PMCID: PMC3529659
- DOI: 10.1161/01.res.66.5.1328
Molecular and particulate depositions for regional myocardial flows in sheep
Abstract
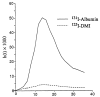
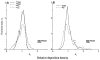
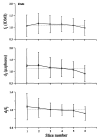
The deposition of microspheres in small tissue regions is not strictly flow dependent. In comparison with the soluble flow marker 2-iododesmethylimipramine (IDMI), deposition of 16.5-microns microspheres was mildly but systematically biased into high flow regions of rabbit hearts (Bassingthwaighte JB, Malone MA, Moffett T-C, King RB, Little SE, Link JM, Krohn KA. Am J Physiol 1987;253 (Heart Circ Physiol 22):H184-H193). To examine the possibility of bias in larger hearts, a similar study was undertaken in sheep. 141Ce- and 103Ru-labeled 16.5-microns microspheres in one syringe and 125I- and 131I-DMI in another syringe were injected simultaneously into the left atrium of five open-chest sheep while obtaining reference blood samples from the femoral artery. In six other sheep, one microsphere type and one IDMI were used. Hearts were removed 1 minute after injection, cut into approximately 254 pieces averaging 217 mg, and regional deposition densities calculated for each tracer from the isotopic counts. Correlations in the five animals between the two differently labeled IDMIs and between the two microspheres were both greater than or equal to 0.98. In all 11 sheep, scatter plots of microsphere deposition densities versus IDMI densities showed that differences between microspheres and IDMI had substantially more scatter (0.84 less than r less than 0.98) but were not random. Microsphere depositions tended to be lower than IDMI depositions in low flow regions and higher in high flow regions, in accord with the expected bias that at a bifurcation a microsphere is most likely to enter the branch with higher flow. There was less bias ascribable to endomyocardial/epicardial maldistribution. Thus, while microsphere depositions appear to err systematically with respect to flow when the regions of interest are small enough that the diameters of their arterioles are only a few times those of the microspheres, microspheres are, in sheep as in rabbits, adequate for estimating regional flows.
Figures


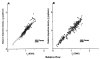
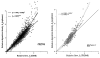




References
-
- King RB, Bassingthwaighte JB. Radioactivity. In: Reneman RS, Strackee J, editors. Data in Medicine. Martinus Nijhoff; The Hague, Netherlands: 1979. pp. 79–113.
-
- Baer RW, Payne BD, Verrier ED, Vlahakes GJ, Molodowitch D, Uhlig PN, Hoffman JIE. Increased number of myocardial blood flow measurements with radionuclide-labeled microspheres. Am J Physiol. 1984;246(15):H418–H434. Heart Circ Physiol. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

