A conserved amphipathic ligand binding region influences k-path-dependent activity of cytochrome C oxidase
- PMID: 23351100
- PMCID: PMC3622084
- DOI: 10.1021/bi3014505
A conserved amphipathic ligand binding region influences k-path-dependent activity of cytochrome C oxidase
Abstract
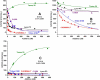
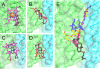
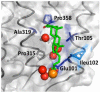
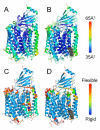
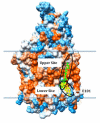
A conserved, crystallographically defined bile acid binding site was originally identified in the membrane domain of mammalian and bacterial cytochrome c oxidase (CcO). Current studies show other amphipathic molecules including detergents, fatty acids, steroids, and porphyrins bind to this site and affect the already 50% inhibited activity of the E101A mutant of Rhodobacter sphaeroides CcO as well as altering the activity of wild-type and bovine enzymes. Dodecyl maltoside, Triton X100, C12E8, lysophophatidylcholine, and CHOBIMALT detergents further inhibit RsCcO E101A, with lesser inhibition observed in wild-type. The detergent inhibition is overcome in the presence of micromolar concentrations of steroids and porphyrin analogues including deoxycholate, cholesteryl hemisuccinate, bilirubin, and protoporphyrin IX. In addition to alleviating detergent inhibition, amphipathic carboxylates including arachidonic, docosahexanoic, and phytanic acids stimulate the activity of E101A to wild-type levels by providing the missing carboxyl group. Computational modeling of dodecyl maltoside, bilirubin, and protoporphyrin IX into the conserved steroid site shows energetically favorable binding modes for these ligands and suggests that a groove at the interface of subunit I and II, including the entrance to the K-path and helix VIII of subunit I, mediates the observed competitive ligand interactions involving two overlapping sites. Spectral analysis indicates that ligand binding to this region affects CcO activity by altering the K-path-dependent electron transfer equilibrium between heme a and heme a(3). The high affinity and specificity of a number of compounds for this region, and its conservation and impact on CcO activity, support its physiological significance.
Figures
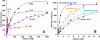
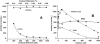
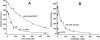

Similar articles
-
The K-path entrance in cytochrome c oxidase is defined by mutation of E101 and controlled by an adjacent ligand binding domain.Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):725-733. doi: 10.1016/j.bbabio.2018.03.017. Epub 2018 Apr 4. Biochim Biophys Acta Bioenerg. 2018. PMID: 29626419 Free PMC article.
-
Computational prediction and in vitro analysis of potential physiological ligands of the bile acid binding site in cytochrome c oxidase.Biochemistry. 2013 Oct 8;52(40):6995-7006. doi: 10.1021/bi400674h. Epub 2013 Sep 27. Biochemistry. 2013. PMID: 24073649 Free PMC article.
-
A conserved steroid binding site in cytochrome C oxidase.Biochemistry. 2008 Sep 23;47(38):9931-3. doi: 10.1021/bi8013483. Epub 2008 Aug 30. Biochemistry. 2008. PMID: 18759498 Free PMC article.
-
Role of conformational change and K-path ligands in controlling cytochrome c oxidase activity.Biochem Soc Trans. 2017 Oct 15;45(5):1087-1095. doi: 10.1042/BST20160138. Epub 2017 Aug 24. Biochem Soc Trans. 2017. PMID: 28842531 Free PMC article. Review.
-
Subunit III-depleted cytochrome c oxidase provides insight into the process of proton uptake by proteins.Biochim Biophys Acta. 2012 Apr;1817(4):545-51. doi: 10.1016/j.bbabio.2011.10.001. Epub 2011 Oct 14. Biochim Biophys Acta. 2012. PMID: 22023935 Free PMC article. Review.
Cited by
-
Interaction of Cytochrome C Oxidase with Steroid Hormones.Cells. 2020 Sep 29;9(10):2211. doi: 10.3390/cells9102211. Cells. 2020. PMID: 33003582 Free PMC article.
-
Complex Interplay of Heme-Copper Oxidases with Nitrite and Nitric Oxide.Int J Mol Sci. 2022 Jan 17;23(2):979. doi: 10.3390/ijms23020979. Int J Mol Sci. 2022. PMID: 35055165 Free PMC article. Review.
-
Characterization and modeling of the oligomeric state and ligand binding behavior of purified translocator protein 18 kDa from Rhodobacter sphaeroides.Biochemistry. 2013 Aug 27;52(34):5884-99. doi: 10.1021/bi400431t. Epub 2013 Aug 16. Biochemistry. 2013. PMID: 23952237 Free PMC article.
-
The mechanism of Intralipid®-mediated cardioprotection complex IV inhibition by the active metabolite, palmitoylcarnitine, generates reactive oxygen species and activates reperfusion injury salvage kinases.PLoS One. 2014 Jan 30;9(1):e87205. doi: 10.1371/journal.pone.0087205. eCollection 2014. PLoS One. 2014. PMID: 24498043 Free PMC article.
-
Monomeric structure of an active form of bovine cytochrome c oxidase.Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):19945-19951. doi: 10.1073/pnas.1907183116. Epub 2019 Sep 18. Proc Natl Acad Sci U S A. 2019. PMID: 31533957 Free PMC article.
References
-
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, ShinzawaItoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 angstrom. Science. 1996;272:1136–1144. - PubMed
-
- Koepke J, Olkhova E, Angerer H, Muller H, Peng G, Michel H. High resolution crystal structure of Paracoccus denitrificans cytochrome c oxidase: new insights into the active site and the proton transfer pathways. Biochim. Biophys. Acta. 2009;1787:635–645. - PubMed
-
- Shinzawa-Itoh K, Aoyama H, Muramoto K, Terada H, Kurauchi T, Tadehara Y, Yamasaki A, Sugimura T, Kurono S, Tsujimoto K, Mizushima T, Yamashita E, Tsukihara T, Yoshikawa S. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007;26:1713–1725. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

