Genistein abrogates G2 arrest induced by curcumin in p53 deficient T47D cells
- PMID: 23351311
- PMCID: PMC3555995
- DOI: 10.1186/2008-2231-20-82
Genistein abrogates G2 arrest induced by curcumin in p53 deficient T47D cells
Abstract
Background: The high cost and low level of cancer survival urge the finding of new drugs having better mechanisms. There is a high trend of patients to be "back to nature" and use natural products as an alternative way to cure cancer. The fact is that some of available anticancer drugs are originated from plants, such as taxane, vincristine, vinblastine, pacitaxel. Curcumin (diferuloylmethane), a dietary pigment present in Curcuma longa rizhome is reported to induce cell cycle arrest in some cell lines. Other study reported that genistein isolated from Glycine max seed inhibited phosphorylation of cdk1, gene involved during G2/M transition and thus could function as G2 checkpoint abrogator. The inhibition of cdk1 phosphorylation is one of alternative strategy which could selectively kill cancer cells and potentially be combined with DNA damaging agent such as curcumin.
Methods: T47D cell line was treated with different concentrations of curcumin and genistein, alone or in combination; added together or with interval time. Flow Cytometry and MTT assay were used to evaluate cell cycle distribution and viability, respectively. The presence of apoptotic cells was determined using acridine orange-ethidium bromide staining.
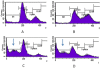
Results: In this study curcumin induced G2 arrest on p53 deficient T47D cells at the concentration of 10 μM. Increasing concentration up to 30 μM increased the number of cell death. Whilst genistein alone at low concentration (≤10 μM) induced cell proliferation, addition of genistein (20 μM) 16 h after curcumin resulted in more cell death (89%), 34% higher than that administered at the same time (56%). The combination treatment resulted in apoptotic cell death. Combining curcumin with high dose of genistein (50 μM) induced necrotic cells.
Conclusions: Genistein increased the death of curcumin treated T47D cells. Appropriate timing of administration and concentration of genistein determine the outcome of treatment and this method could potentially be developed as an alternative strategy for treatment of p53 defective cancer cells.
Figures


Similar articles
-
Role of curcumin in regulating p53 in breast cancer: an overview of the mechanism of action.Breast Cancer (Dove Med Press). 2018 Nov 29;10:207-217. doi: 10.2147/BCTT.S167812. eCollection 2018. Breast Cancer (Dove Med Press). 2018. PMID: 30568488 Free PMC article. Review.
-
Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells.Biomedicines. 2024 Feb 26;12(3):518. doi: 10.3390/biomedicines12030518. Biomedicines. 2024. PMID: 38540131 Free PMC article.
-
Down-regulation of Cdc25c, CDK1 and Cyclin B1 and Up-regulation of Wee1 by Curcumin Promotes Human Colon Cancer Colo 205 Cell Entry into G2/M-phase of Cell Cycle.Cancer Genomics Proteomics. 2006 Jan-Feb;3(1):55-61. Epub 2006 Jan 1. Cancer Genomics Proteomics. 2006. PMID: 31394642
-
UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53.J Natl Cancer Inst. 1996 Jul 17;88(14):956-65. doi: 10.1093/jnci/88.14.956. J Natl Cancer Inst. 1996. PMID: 8667426
-
Curcumin prevented human autocrine growth hormone (GH) signaling mediated NF-κB activation and miR-183-96-182 cluster stimulated epithelial mesenchymal transition in T47D breast cancer cells.Mol Biol Rep. 2019 Feb;46(1):355-369. doi: 10.1007/s11033-018-4479-y. Epub 2018 Nov 23. Mol Biol Rep. 2019. PMID: 30467667
Cited by
-
Role of curcumin in regulating p53 in breast cancer: an overview of the mechanism of action.Breast Cancer (Dove Med Press). 2018 Nov 29;10:207-217. doi: 10.2147/BCTT.S167812. eCollection 2018. Breast Cancer (Dove Med Press). 2018. PMID: 30568488 Free PMC article. Review.
-
Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells.Biomedicines. 2024 Feb 26;12(3):518. doi: 10.3390/biomedicines12030518. Biomedicines. 2024. PMID: 38540131 Free PMC article.
-
Selective induced apoptosis and cell cycle arrest in MCF7 and LNCap cell lines by skin mucus from round goby (Neogobius melanostomus) and common carp (Cyprinus carpio) through P53 expression.Cytotechnology. 2020 Jun;72(3):367-376. doi: 10.1007/s10616-020-00383-x. Epub 2020 Mar 6. Cytotechnology. 2020. PMID: 32144633 Free PMC article.
-
Selective induction of DNA damage, G2 abrogation, and mitochondrial apoptosis by leaf extract of traditional medicinal plant Wrightia arborea in K562 cells.Protoplasma. 2018 Jan;255(1):203-216. doi: 10.1007/s00709-017-1137-5. Epub 2017 Jul 20. Protoplasma. 2018. PMID: 28730515
References
-
- Wang Y, Li J, Booher RN, Kraker A, Lawrence T, Leopold WR, Sun Y. Radiosensitization of p53 Mutant Cells by PD0166285, a Novel G2 Checkpoint Abrogator. Cancer Res. 2001;61:8211–8217. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

