Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy
- PMID: 23351389
- PMCID: PMC3563497
- DOI: 10.1186/1479-5876-11-21
Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy
Abstract
Background: Stem cell therapy is a promising treatment for cerebral palsy, which refers to a category of brain diseases that are associated with chronic motor disability in children. Autologous MSCs may be a better cell source and have been studied for the treatment of cerebral palsy because of their functions in tissue repair and the regulation of immunological processes.
Methods: To assess neural stem cell-like (NSC-like) cells derived from autologous marrow mesenchymal stem cells as a novel treatment for patients with moderate-to-severe cerebral palsy, a total of 60 cerebral palsy patients were enrolled in this open-label, non-randomised, observer-blinded controlled clinical study with a 6-months follow-up. For the transplantation group, a total of 30 cerebral palsy patients received an autologous NSC-like cells transplantation (1-2 × 107 cells into the subarachnoid cavity) and rehabilitation treatments whereas 30 patients in the control group only received rehabilitation treatment.
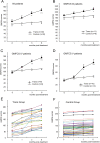
Results: We recorded the gross motor function measurement scores, language quotients, and adverse events up to 6 months post-treatment. The gross motor function measurement scores in the transplantation group were significantly higher at month 3 (the score increase was 42.6, 95% CI: 9.8-75.3, P=.011) and month 6 (the score increase was 58.6, 95% CI: 25.8-91.4, P=.001) post-treatment compared with the baseline scores. The increase in the Gross Motor Function Measurement scores in the control group was not significant. The increases in the language quotients at months 1, 3, and 6 post-treatment were not statistically significant when compared with the baseline quotients in both groups. All the 60 patients survived, and none of the patients experienced serious adverse events or complications.
Conclusion: Our results indicated that NSC-like cells are safe and effective for the treatment of motor deficits related to cerebral palsy. Further randomised clinical trials are necessary to establish the efficacy of this procedure.
Figures



Similar articles
-
Administration of Autologous Bone Marrow-Derived Stem Cells for Treatment of Cerebral Palsy Patients: A Proof of Concept.J Stem Cells. 2016;11(1):37-49. J Stem Cells. 2016. PMID: 28296863 Clinical Trial.
-
Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy.J Transl Med. 2017 Feb 24;15(1):48. doi: 10.1186/s12967-017-1149-0. J Transl Med. 2017. PMID: 28235424 Free PMC article. Clinical Trial.
-
Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: a preliminary clinical study.Cytotherapy. 2013 Dec;15(12):1549-62. doi: 10.1016/j.jcyt.2013.06.001. Epub 2013 Oct 5. Cytotherapy. 2013. PMID: 24100132 Clinical Trial.
-
Systematic review of controlled clinical studies using umbilical cord blood for regenerative therapy: Identifying barriers to assessing efficacy.Cytotherapy. 2019 Nov;21(11):1112-1121. doi: 10.1016/j.jcyt.2019.08.004. Epub 2019 Oct 3. Cytotherapy. 2019. PMID: 31587876
-
Stem Cell Treatment and Cerebral Palsy: A Systematic Review and Meta-Analysis.Curr Stem Cell Res Ther. 2024;19(2):210-219. doi: 10.2174/1574888X18666221201114756. Curr Stem Cell Res Ther. 2024. PMID: 36464870
Cited by
-
Improved Quality of Life in A Case of Cerebral Palsy after Bone Marrow Mononuclear Cell Transplantation.Cell J. 2015 Summer;17(2):389-94. doi: 10.22074/cellj.2016.3754. Epub 2015 Jul 11. Cell J. 2015. PMID: 26199918 Free PMC article.
-
Cell Therapy for Neurological Disorders: The Perspective of Promising Cells.Biology (Basel). 2021 Nov 6;10(11):1142. doi: 10.3390/biology10111142. Biology (Basel). 2021. PMID: 34827135 Free PMC article. Review.
-
Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study.Stem Cells Int. 2013;2013:623875. doi: 10.1155/2013/623875. Epub 2013 Aug 25. Stem Cells Int. 2013. PMID: 24062774 Free PMC article.
-
Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy.J Transl Med. 2014 Dec 12;12:318. doi: 10.1186/s12967-014-0318-7. J Transl Med. 2014. PMID: 25496119 Free PMC article.
-
Self-renewal of neural stem cells: implications for future therapies.Front Physiol. 2013 Mar 18;4:49. doi: 10.3389/fphys.2013.00049. eCollection 2013. Front Physiol. 2013. PMID: 23513073 Free PMC article. No abstract available.
References
-
- An T, Guo XQ, Pu XH. Study progress in early intervention of high- risk infants for the prevention and treatment of cerebral palsy. Clin Pediatr. 2006;24:696–698.
-
- Hu YM. Jiang ZF. Zhu FT: Practice of pediatrics. People’s medical publishing house; 2002.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

