NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials
- PMID: 23351618
- PMCID: PMC3610248
- DOI: 10.1186/1742-6405-10-5
NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials
Abstract
Background: HIV-associated distal sensory polyneuropathy (HIV-DSP) is the most frequently reported neurologic complication associated with HIV infection. NGX-4010 is a capsaicin 8% dermal patch with demonstrated efficacy in the treatment of HIV-DSP. Data from two phase III, double-blind studies were integrated to further analyze the efficacy and safety of NGX-4010 and explore the effect of demographic and baseline factors on NGX-4010 treatment in HIV-DSP.
Methods: Data from two similarly designed studies in which patients with HIV-DSP received NGX-4010 or a low-concentration control patch (capsaicin 0.04% w/w) for 30 or 60 minutes were integrated. Efficacy assessments included the mean percent change from baseline in Numeric Pain Rating Scale (NPRS) scores to Weeks 2-12. Safety and tolerability assessments included adverse events (AEs) and pain during and after treatment.
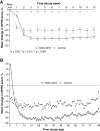
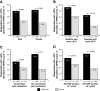
Results: Patients (n = 239) treated with NGX-4010 for 30 minutes demonstrated significantly (p = 0.0026) greater pain relief compared with controls (n = 100); the mean percent change in NPRS scores from baseline to Weeks 2-12 was -27.0% versus -15.7%, respectively. Patients who received a 60-minute application of NGX-4010 (n = 243) showed comparable pain reductions (-27.5%) to patients treated for 30 minutes, but this was not statistically superior to controls (n = 115). NGX-4010 was effective regardless of gender, baseline pain score, duration of HIV-DSP, or use of concomitant neuropathic pain medication, although NGX-4010 efficacy was greater in patients not receiving concomitant neuropathic pain medications. NGX-4010 was well tolerated; the most common AEs were application-site pain and erythema, and most AEs were mild to moderate. The transient increase in pain associated with NGX-4010 treatment decreased the day after treatment and returned to baseline by Day 2.
Conclusions: A single 30-minute application of NGX-4010 provides significant pain relief for at least 12 weeks in patients with HIV-DSP and is well tolerated.
Trial registration: C107 = NCT00064623; C119 = NCT00321672.
Figures

References
-
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. - DOI - PMC - PubMed
-
- Kieburtz K, Simpson D, Yiannoutsos C, Max MB, Hall CD, Ellis RJ, Marra CM, McKendall R, Singer E, Dal Pan GJ, Clifford DB, Tucker T, Cohen B. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. AIDS clinical trial group 242 protocol team. Neurology. 1998;51:1682–1688. doi: 10.1212/WNL.51.6.1682. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

