Positive effect of septimeb™ on mortality rate in severe sepsis: a novel non antibiotic strategy
- PMID: 23351964
- PMCID: PMC3555989
- DOI: 10.1186/2008-2231-20-40
Positive effect of septimeb™ on mortality rate in severe sepsis: a novel non antibiotic strategy
Abstract
Background: Septimeb is a new herbal-derived remedy, recently approved for its potential immunomodulatory effects. Regarding the key role of immune system in the pathogenesis of severe sepsis and lack of any standard treatment for improving survival of these patients; we evaluated the effect of Septimeb -as an adjutant to standard treatment-on inflammatory biomarkers and mortality rates in patients with severe sepsis.
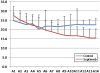
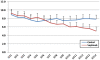
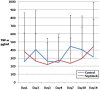
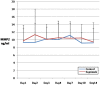
Methods: In this multicenter, randomized, single-blind trial, we assigned patients with severe sepsis and Acute Physiology and Chronic Health Evaluation (APACHE II) score of more than 20 to receive standard treatment of severe sepsis (control group) or standard treatment plus Septimeb. This group was treated with Septimeb for 14 days then followed up for another14 days. APACHE score, Sequential Organ Failure Assessment (SOFA) and Simplified Acute Physiology Score (SAPS) were calculated daily. Blood samples were analyzed for interleukin 2 tumor necrosis factor-α, total antioxidant power, platelet growth factor and matrix metalloproteinase 2.
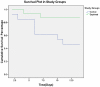
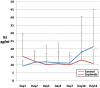
Results: A total of 29 patients underwent randomization (13 in control group and 16 in Septimeb group). There was significant difference between the Septimeb and control group in the 14 days mortality rate (18.8% vs. 53.85 respectively, P=0.048). Compared to control group, Septimeb was significantly effective in improving SAPS (P= 0.029), SOFA (P=0.003) and APACHE II (P=0.008) scores. Inflammatory biomarkers didn't change significantly between the two groups (P>0.05).
Conclusion: Septimeb reduces mortality rates among patients with severe sepsis and it could be added as a safe adjutant to standard treatment of sepsis.
Figures



References
-
- Mousavi S, Mojtahedzadeh M, Abdollahi M. Place of iron chelators like desferrioxamine and deferasirox in management of hyperoxia-induced lung injury: A systematic review. Int J Pharmacol. 2010;6:326–337.
LinkOut - more resources
Full Text Sources

