A bacterial source for mollusk pyrone polyketides
- PMID: 23352141
- PMCID: PMC3558931
- DOI: 10.1016/j.chembiol.2012.10.019
A bacterial source for mollusk pyrone polyketides
Abstract
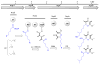
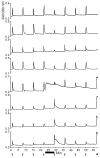
In the oceans, secondary metabolites often protect otherwise poorly defended invertebrates, such as shell-less mollusks, from predation. The origins of these metabolites are largely unknown, but many of them are thought to be made by symbiotic bacteria. In contrast, mollusks with thick shells and toxic venoms are thought to lack these secondary metabolites because of reduced defensive needs. Here, we show that heavily defended cone snails also occasionally contain abundant secondary metabolites, γ-pyrones known as nocapyrones, which are synthesized by symbiotic bacteria. The bacteria, Nocardiopsis alba CR167, are related to widespread actinomycetes that we propose to be casual symbionts of invertebrates on land and in the sea. The natural roles of nocapyrones are unknown, but they are active in neurological assays, revealing that mollusks with external shells are an overlooked source of secondary metabolite diversity.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




References
-
- Benkendorff K. Molluscan biological and chemical diversity: secondary metabolites and medicinal resources produced by marine molluscs. Biol Rev Cambridge Phil Soc. 2010;85:757–775. - PubMed
-
- Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2011;28:196–268. - PubMed
-
- Busch B, Hertweck C. Evolution of metabolic diversity in polyketide-derived pyrones: Using the noncolinear aureothin assembly line as a model system. Phytochemistry. 2009;70:1833–1840. - PubMed
-
- Cimino G, Sodano G, Spinella A. New propionate-derived metabolites from Aglaja depicta and from its prey Bulla striata (opisthobranch mollusks) J Org Chem. 1987;52:5326–5331.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

