Effector kinase coupling enables high-throughput screens for direct HIV-1 Nef antagonists with antiretroviral activity
- PMID: 23352142
- PMCID: PMC3559019
- DOI: 10.1016/j.chembiol.2012.11.005
Effector kinase coupling enables high-throughput screens for direct HIV-1 Nef antagonists with antiretroviral activity
Abstract
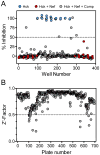
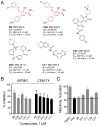
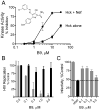
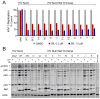
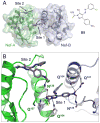
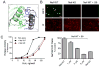
HIV-1 Nef, a critical AIDS progression factor, represents an important target protein for antiretroviral drug discovery. Because Nef lacks intrinsic enzymatic activity, we developed an assay that couples Nef to the activation of Hck, a Src family member and Nef effector protein. Using this assay, we screened a large, diverse chemical library and identified small molecules that block Nef-dependent Hck activity with low micromolar potency. Of these, a diphenylpyrazolo compound demonstrated submicromolar potency in HIV-1 replication assays against a broad range of primary Nef variants. This compound binds directly to Nef via a pocket formed by the Nef dimerization interface and disrupts Nef dimerization in cells. Coupling of nonenzymatic viral accessory factors to host cell effector proteins amenable to high-throughput screening may represent a general strategy for the discovery of new antimicrobial agents.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Chemical library screens targeting an HIV-1 accessory factor/host cell kinase complex identify novel antiretroviral compounds.ACS Chem Biol. 2009 Nov 20;4(11):939-47. doi: 10.1021/cb900195c. ACS Chem Biol. 2009. PMID: 19807124 Free PMC article.
-
Subtle Dynamic Changes Accompany Hck Activation by HIV-1 Nef and are Reversed by an Antiretroviral Kinase Inhibitor.Biochemistry. 2015 Oct 20;54(41):6382-91. doi: 10.1021/acs.biochem.5b00875. Epub 2015 Oct 6. Biochemistry. 2015. PMID: 26440750 Free PMC article.
-
Tight-Binding Hydroxypyrazole HIV-1 Nef Inhibitors Suppress Viral Replication in Donor Mononuclear Cells and Reverse Nef-Mediated MHC-I Downregulation.ACS Infect Dis. 2020 Feb 14;6(2):302-312. doi: 10.1021/acsinfecdis.9b00382. Epub 2019 Dec 16. ACS Infect Dis. 2020. PMID: 31775511 Free PMC article.
-
Structure, function, and inhibitor targeting of HIV-1 Nef-effector kinase complexes.J Biol Chem. 2020 Oct 30;295(44):15158-15171. doi: 10.1074/jbc.REV120.012317. Epub 2020 Aug 29. J Biol Chem. 2020. PMID: 32862141 Free PMC article. Review.
-
Small molecule inhibitors of the HIV-1 virulence factor, Nef.Drug Discov Today Technol. 2013 Dec;10(4):e523-9. doi: 10.1016/j.ddtec.2013.07.002. Drug Discov Today Technol. 2013. PMID: 24451644 Free PMC article. Review.
Cited by
-
Lovastatin Inhibits HIV-1-Induced MHC-I Downregulation by Targeting Nef-AP-1 Complex Formation: A New Strategy to Boost Immune Eradication of HIV-1 Infected Cells.Front Immunol. 2019 Sep 10;10:2151. doi: 10.3389/fimmu.2019.02151. eCollection 2019. Front Immunol. 2019. PMID: 31572371 Free PMC article.
-
Review of Current Cell-Penetrating Antibody Developments for HIV-1 Therapy.Molecules. 2018 Feb 6;23(2):335. doi: 10.3390/molecules23020335. Molecules. 2018. PMID: 29415435 Free PMC article. Review.
-
Dynamics of the Tec-family tyrosine kinase SH3 domains.Protein Sci. 2016 Apr;25(4):852-64. doi: 10.1002/pro.2887. Epub 2016 Mar 18. Protein Sci. 2016. PMID: 26808198 Free PMC article.
-
Inhibitors of HIV-1 Nef-Mediated Activation of the Myeloid Src-Family Kinase Hck Block HIV-1 Replication in Macrophages and Disrupt MHC-I Downregulation.ACS Infect Dis. 2022 Jan 14;8(1):91-105. doi: 10.1021/acsinfecdis.1c00288. Epub 2022 Jan 5. ACS Infect Dis. 2022. PMID: 34985256 Free PMC article.
-
A single β-octyl glucoside molecule induces HIV-1 Nef dimer formation in the absence of partner protein binding.PLoS One. 2018 Feb 7;13(2):e0192512. doi: 10.1371/journal.pone.0192512. eCollection 2018. PLoS One. 2018. PMID: 29415006 Free PMC article.
References
-
- Arold ST, Baur AS. Dynamic Nef and Nef dynamics: how structure could explain the complex activities of this small HIV protein. Trends Biochem Sci. 2001;26:356–363. - PubMed
-
- Atkins KM, Thomas L, Youker RT, Harriff MJ, Pissani F, You H, Thomas G. HIV-1 Nef binds PACS-2 to assemble a multikinase cascade that triggers major histocompatibility complex class I (MHC-I) down-regulation: analysis using short interfering RNA and knock-out mice. J Biol Chem. 2008;283:11772–11784. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

