Vulnerable neural systems and the borderland of brain aging and neurodegeneration
- PMID: 23352159
- PMCID: PMC3558930
- DOI: 10.1016/j.neuron.2013.01.002
Vulnerable neural systems and the borderland of brain aging and neurodegeneration
Abstract
Brain aging is characterized by considerable heterogeneity, including varying degrees of dysfunction in specific brain systems, notably a medial temporal lobe memory system and a frontostriatal executive system. These same systems are also affected by neurodegenerative diseases. Recent work using techniques for presymptomatic detection of disease in cognitively normal older people has shown that some of the late life alterations in cognition, neural structure, and function attributed to aging probably reflect early neurodegeneration. However, it has become clear that these same brain systems are also vulnerable to aging in the absence of even subtle disease. Thus, fundamental systemic limitations appear to confer vulnerability of these neural systems to a variety of insults, including those recognized as typical disease and those that are attributed to age. By focusing on the fundamental causes of neural system vulnerability, the prevention or treatment of a wide range of late-life neural dysfunction might be possible.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


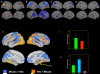
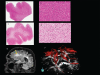
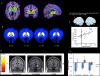
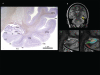
References
-
- Aarsland D, Tandberg E, Larsen JP, Cummings JL. Frequency of dementia in Parkinson disease. Arch Neurol. 1996;53:538–542. - PubMed
-
- Alexander GE, DeLongh RM, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. - PubMed
-
- Amaral DG. Morphological analyses of the brains of behaviorally characterized aged nonhuman primates. Neurobiol Aging. 1993;14:671–672. - PubMed
-
- Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

