Risk-responsive orbitofrontal neurons track acquired salience
- PMID: 23352162
- PMCID: PMC3559000
- DOI: 10.1016/j.neuron.2012.11.006
Risk-responsive orbitofrontal neurons track acquired salience
Abstract
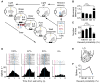
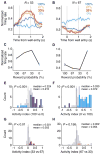
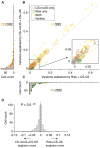
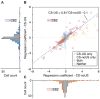
Decision making is impacted by uncertainty and risk (i.e., variance). Activity in the orbitofrontal cortex, an area implicated in decision making, covaries with these quantities. However, this activity could reflect the heightened salience of situations in which multiple outcomes-reward and reward omission-are expected. To resolve these accounts, rats were trained to respond to cues predicting 100%, 67%, 33%, or 0% reward. Consistent with prior reports, some orbitofrontal neurons fired differently in anticipation of uncertain (33% and 67%) versus certain (100% and 0%) reward. However, over 90% of these neurons also fired differently prior to 100% versus 0% reward (or baseline) or prior to 33% versus 67% reward. These responses are inconsistent with risk but fit well with the representation of acquired salience linked to the sum of cue-outcome and cue-no-outcome associative strengths. These results expand our understanding of how the orbitofrontal cortex might regulate learning and behavior.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Bach DR, Dolan RJ. Knowing how much you don’t know: a neural organization of uncertainty estimates. Nature Reviews Neuroscience. 2012;13:572–586. - PubMed
-
- Chase EA, Tait DS, Brown VJ. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. European Journal of Neuroscience. 2012;36:2368–2375. - PubMed
-
- D’Acremont M, Bossaerts P. Neurobiological studies of risk assessment: a comparison of expected utility and mean-variance approaches. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:363–374. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

