Synaptic vesicles position complexin to block spontaneous fusion
- PMID: 23352168
- PMCID: PMC3559010
- DOI: 10.1016/j.neuron.2012.11.005
Synaptic vesicles position complexin to block spontaneous fusion
Abstract
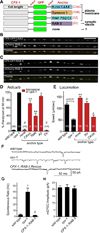
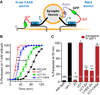
Synapses continually replenish their synaptic vesicle (SV) pools while suppressing spontaneous fusion events, thus maintaining a high dynamic range in response to physiological stimuli. The presynaptic protein complexin can both promote and inhibit fusion through interactions between its α-helical domain and the SNARE complex. In addition, complexin's C-terminal half is required for the inhibition of spontaneous fusion in worm, fly, and mouse, although the molecular mechanism remains unexplained. We show here that complexin's C-terminal domain binds lipids through a novel protein motif, permitting complexin to inhibit spontaneous exocytosis in vivo by targeting complexin to SVs. We propose that the SV pool serves as a platform to sequester and position complexin where it can intercept the rapidly assembling SNAREs and control the rate of spontaneous fusion.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Bracher A, Kadlec J, Betz H, Weissenhorn W. X-ray structure of a neuronal complexin-SNARE complex from squid. J Biol Chem. 2002;277:26517–26523. - PubMed
-
- Brose N. For Better or for Worse: Complexins Regulate SNARE Function and Vesicle Fusion. Traffic. 2008 - PubMed
-
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002;35:107–120. - PubMed
-
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

