Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study
- PMID: 23352199
- PMCID: PMC3684942
- DOI: 10.1016/S1474-4422(12)70323-X
Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study
Abstract
Background: Many women of childbearing potential take antiepileptic drugs, but the cognitive effects of fetal exposure are uncertain. We aimed to assess effects of commonly used antiepileptic drugs on cognitive outcomes in children up to 6 years of age.
Methods: In this prospective, observational, assessor-masked, multicentre study, we enrolled pregnant women with epilepsy on antiepileptic drug monotherapy (carbamazepine, lamotrigine, phenytoin, or valproate) between October, 1999, and February, 2004, at 25 epilepsy centres in the UK and the USA. Our primary outcome was intelligence quotient (IQ) at 6 years of age (age-6 IQ) in all children, assessed with linear regression adjusted for maternal IQ, antiepileptic drug type, standardised dose, gestational birth age, and use of periconceptional folate. We also assessed multiple cognitive domains and compared findings with outcomes at younger ages. This study is registered with ClinicalTrials.gov, number NCT00021866.
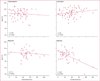
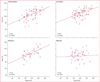
Findings: We included 305 mothers and 311 children (six twin pairs) in the primary analysis. 224 children completed 6 years of follow-up (6-year-completer sample). Multivariate analysis of all children showed that age-6 IQ was lower after exposure to valproate (mean 97, 95% CI 94-101) than to carbamazepine (105, 102-108; p=0·0015), lamotrigine (108, 105-110; p=0·0003), or phenytoin (108, 104-112; p=0·0006). Children exposed to valproate did poorly on measures of verbal and memory abilities compared with those exposed to the other antiepileptic drugs and on non-verbal and executive functions compared with lamotrigine (but not carbamazepine or phenytoin). High doses of valproate were negatively associated with IQ (r=-0·56, p<0·0001), verbal ability (r=-0·40, p=0·0045), non-verbal ability (r=-0·42, p=0·0028), memory (r=-0·30, p=0·0434), and executive function (r=-0·42, p=0·0004), but other antiepileptic drugs were not. Age-6 IQ correlated with IQs at younger ages, and IQ improved with age for infants exposed to any antiepileptic drug. Compared with a normative sample (173 [93%] of 187 children), right-handedness was less frequent in children in our study overall (185 [86%] of 215; p=0·0404) and in the lamotrigine (59 [83%] of 71; p=0·0287) and valproate (38 [79%] of 40; p=0·0089) groups. Verbal abilities were worse than non-verbal abilities in children in our study overall and in the lamotrigine and valproate groups. Mean IQs were higher in children exposed to periconceptional folate (108, 95% CI 106-111) than they were in unexposed children (101, 98-104; p=0·0009).
Interpretation: Fetal valproate exposure has dose-dependent associations with reduced cognitive abilities across a range of domains at 6 years of age. Reduced right-handedness and verbal (vs non-verbal) abilities might be attributable to changes in cerebral lateralisation induced by exposure to antiepileptic drugs. The positive association of periconceptional folate with IQ is consistent with other recent studies.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Antiepileptic drugs during pregnancy and cognitive outcomes.Lancet Neurol. 2013 Mar;12(3):219-20. doi: 10.1016/S1474-4422(13)70012-7. Epub 2013 Jan 23. Lancet Neurol. 2013. PMID: 23352200 No abstract available.
References
-
- Schwarz EB, Maselli J, Norton M, Gonzales R. Prescription of teratogenic medications in United States ambulatory practices. Am J Med. 2005;118:1240–1249. - PubMed
-
- Harden CL, Meador KJ, Pennell PB, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis, perinatal outcomes Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:133–141. - PMC - PubMed
-
- US FDA. [accessed June 23, 2012];FDA drug safety communication: children born to mothers who took Valproate products while pregnant may have impaired cognitive development. http://www.fda.gov/Drugs/DrugSafety/ucm261543.htm.
-
- Sattler JM. Assessment of children: cognitive foundations. 5th edn. San Diego, CA: Jerome M Sattler; 2008.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

