Treatment complexity in cystic fibrosis: trends over time and associations with site-specific outcomes
- PMID: 23352205
- PMCID: PMC4073628
- DOI: 10.1016/j.jcf.2012.12.009
Treatment complexity in cystic fibrosis: trends over time and associations with site-specific outcomes
Abstract
Background: Patients with cystic fibrosis (CF) have increasing treatment complexity and high treatment burden. We describe trends in treatment complexity and evaluate its relationship with health outcomes.
Methods: Using Epidemiologic Study of Cystic Fibrosis (ESCF) data, we developed a treatment complexity score (TCS) from 37 chronic therapies and assessed change by age group (6-13, 14-17, and 18+ years) over a three year period. Differences in average site TCS were evaluated by quartiles based on FEV1, BMI, or Treatment Burden score on the Cystic Fibrosis Questionnaire-Revised (CFQ-R).
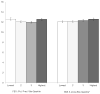
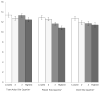
Results: TCS scores were calculated for 7252 individual patients (42% child, 16% adolescent, 43% adult) across 153 sites. In 2003, mean TCS was 11.1 for children, 11.8 for adolescents, and 12.1 for adults. In all 3 age groups, TCS increased over 3 years; the increase in TCS from 2003-2005 for children was 1.25 (95% CI 1.16-1.34), for adolescents 0.77 (0.62-0.93), and for adults 1.20 (1.08-1.31) (all P<0.001 for trend over time). At the site level, there were no significant differences in mean TCS based on FEV1 quartile. Mean TCS was higher in the highest BMI z-score quartile. Across all 3 versions of the CFQ-R, mean TCS was lower at sites in the highest quartiles (lowest burden) for CFQ-R treatment burden scores.
Conclusion: Treatment complexity was highest among adults with CF, although over 3 years, we observed a significant increase in treatment complexity in all age groups. Such increases in treatment complexity pose a challenge to patient self-management and adherence. Future research is needed to understand the associations between treatment complexity and subsequent health outcomes to reduce treatment burden and improve disease management.
Copyright © 2013 European Cystic Fibrosis Society. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Disclosure of Conflict of Interest
Gregory Sawicki, Clement Ren, Michael Konstan, and Alexandra Quittner have received honoraria from Genentech for serving as members of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF) and have served as consultants to Genentech. No compensation was provided to these authors in exchange for production of this manuscript. Stefanie Millar and David Pasta are employees of ICON Late Phase & Outcomes Research, which was paid by Genentech for providing analytical services for this study.
Figures

References
-
- Foundation CF. Cystic Fibrosis Foundation Patient Registry Annual Data Report 2010. Bethesda, Maryland: Cystic Fibrosis Foundation; 2011.
-
- Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. American journal of respiratory and critical care medicine. 2007;176(10):957–969. - PubMed
-
- Flume PA, Robinson KA, O’Sullivan BP, Finder JD, Vender RL, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54(4):522–537. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

