Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era
- PMID: 23352568
- PMCID: PMC3713786
- DOI: 10.1016/j.breast.2012.12.006
Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era
Abstract
Background: Trastuzumab is associated with improvements in overall survival (OS) among patients with HER2-positive metastatic breast cancer (MBC); however disease course and patterns of care in individual patients are highly variable.
Methods: 113 HER2-positive patients diagnosed with MBC from 1999 to 2005 who received trastuzumab-based therapy were retrospectively identified to allow for a minimum of 5 years of follow-up time. Median OS and median duration of therapy were determined using Kaplan-Meier methodology and group comparisons were based on the log-rank test. Hazard ratios (HR) were obtained using a Cox proportional hazards model.
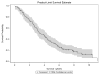
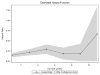
Results: Median OS was 3.5 years (95% CI 3.0-4.4) from time of initiation of first therapy in the metastatic setting. On univariate analysis, central nervous system (CNS) disease at first recurrence was associated with a shorter OS compared with liver and/or lung metastases or other sites (CNS: 1.9 years CI 0.1-5.9, liver/lung: 3.2 years CI 2.5-4.2, other: 4.6 years CI 2.7-8.0; p = 0.05), however, this was not predictive of survival outcome in multivariate analysis. CNS metastases developed in 62 (55%) patients by the time of death or last follow-up. Median duration of therapy was similar up to 6 lines of treatment, and ranged from 5.2 months to 7.2 months.
Conclusions: The natural history of HER2-positive MBC has evolved with trastuzumab-based therapy with median OS now exceeding 3 years. CNS disease is a major problem with continued risk of CNS progression over time. Patients demonstrate clinical benefit to multiple lines of HER2-directed therapy.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All other authors have no conflicts of interest.
Figures


References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. - PubMed
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. - PubMed
-
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. - PubMed
-
- Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2001;19(10):2722–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

