Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography
- PMID: 23352648
- PMCID: PMC3616770
- DOI: 10.1016/j.jbiomech.2012.12.024
Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography
Abstract
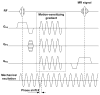
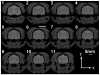
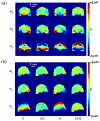
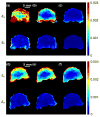
Characterization of the dynamic mechanical behavior of brain tissue is essential for understanding and simulating the mechanisms of traumatic brain injury (TBI). Changes in mechanical properties may also reflect changes in the brain due to aging or disease. In this study, we used magnetic resonance elastography (MRE) to measure the viscoelastic properties of ferret brain tissue in vivo. Three-dimensional (3D) displacement fields were acquired during wave propagation in the brain induced by harmonic excitation of the skull at 400 Hz, 600 Hz and 800 Hz. Shear waves with wavelengths in the order of millimeters were clearly visible in the displacement field, in strain fields, and in the curl of displacement field (which contains no contributions from longitudinal waves). Viscoelastic parameters (storage and loss moduli) governing dynamic shear deformation were estimated in gray and white matter for these excitation frequencies. To characterize the reproducibility of measurements, two ferrets were studied on three different dates each. Estimated viscoelastic properties of white matter in the ferret brain were generally similar to those of gray matter and consistent between animals and scan dates. In both tissue types G' increased from approximately 3 kPa at 400 Hz to 7 kPa at 800 Hz and G″ increased from approximately 1 kPa at 400 Hz to 2 kPa at 800 Hz. These measurements of shear wave propagation in the ferret brain can be used to both parameterize and validate finite element models of brain biomechanics.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors has a conflict of interest that could influence the work described in this manuscript.
Figures





References
-
- Asbach P, Klatt D, Hamhaber U, Braun J, Somasundaram R, Hamm B, Sack I. Assessment of liver viscoelasticity using multifrequency MR elastography. Magn Reson Med. 2008;60:373–379. - PubMed
-
- Auld BA. Acoustic fields and waves in solids. 2. R.E. Krieger; Malabar, FL: 1990.
-
- Boulet T, Kelso ML, Othman SF. Microscopic magnetic resonance elastography of traumatic brain injury model. J Neurosci Methods. 2011;201:296–306. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

