Polyproline tetramer organizing peptides in fetal bovine serum acetylcholinesterase
- PMID: 23352838
- PMCID: PMC3594448
- DOI: 10.1016/j.bbapap.2013.01.009
Polyproline tetramer organizing peptides in fetal bovine serum acetylcholinesterase
Abstract
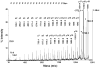
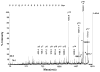
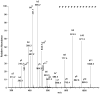
Acetylcholinesterase (AChE) in the serum of fetal cow is a tetramer. The related enzyme, butyrylcholinesterase (BChE), in the sera of humans and horse requires polyproline peptides for assembly into tetramers. Our goal was to determine whether soluble tetrameric AChE includes tetramer organizing peptides in its structure. Fetal bovine serum AChE was denatured by boiling to release non-covalently bound peptides. Bulk protein was separated from peptides by filtration and by high performance liquid chromatography. Peptide mass and amino acid sequence of the released peptides were determined by MALDI-TOF-TOF and LTQ-Orbitrap mass spectrometry. Twenty polyproline peptides, divided into 5 families, were identified. The longest peptide contained 25 consecutive prolines and no other amino acid. Other polyproline peptides included one non-proline amino acid, for example serine at the C-terminus of 20 prolines. A search of the mammalian proteome database suggested that this assortment of polyproline peptides originated from at least 5 different precursor proteins, none of which were the ColQ or PRiMA of membrane-anchored AChE. To date, AChE and BChE are the only proteins known that include polyproline tetramer organizing peptides in their tetrameric structure.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors have any conflict of interest or potential conflict of interest regarding this paper.
Figures



References
-
- Massoulie J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993;41:31–91. - PubMed
-
- Li B, Stribley JA, Ticu A, Xie W, Schopfer LM, Hammond P, Brimijoin S, Hinrichs SH, Lockridge O. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J Neurochem. 2000;75:1320–1331. - PubMed
-
- Perrier AL, Massoulie J, Krejci E. PRiMA: the membrane anchor of acetylcholinesterase in the brain. Neuron. 2002;33:275–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

