Adenylylation of mycobacterial Glnk (PII) protein is induced by nitrogen limitation
- PMID: 23352854
- PMCID: PMC3612183
- DOI: 10.1016/j.tube.2012.12.003
Adenylylation of mycobacterial Glnk (PII) protein is induced by nitrogen limitation
Abstract
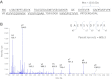
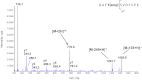
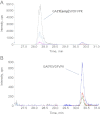
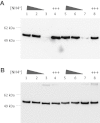
PII proteins are pivotal regulators of nitrogen metabolism in most prokaryotes, controlling the activities of many targets, including nitrogen assimilation enzymes, two component regulatory systems and ammonium transport proteins. Escherichia coli contains two PII-like proteins, PII (product of glnB) and GlnK, both of which are uridylylated under nitrogen limitation at a conserved Tyrosine-51 residue by GlnD (a uridylyl transferase). PII-uridylylation in E. coli controls glutamine synthetase (GS) adenylylation by GlnE and mediates the NtrB/C transcriptomic response. Mycobacteria contain only one PII protein (GlnK) which in environmental Actinomycetales is adenylylated by GlnD under nitrogen limitation. However in mycobacteria, neither the type of GlnK (PII) covalent modification nor its precise role under nitrogen limitation is known. In this study, we used LC-Tandem MS to analyse the modification state of mycobacterial GlnK (PII), and demonstrate that during nitrogen limitation GlnK from both non-pathogenic Mycobacterium smegmatis and pathogenic Mycobacterium tuberculosis is adenylylated at the Tyrosine-51 residue; we also show that GlnD is the adenylyl transferase enzyme responsible. Further analysis shows that in contrast to E. coli, GlnK (PII) adenylylation in M. tuberculosis does not regulate GS adenylylation, nor does it mediate the transcriptomic response to nitrogen limitation.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Ninfa A.J., Jiang P. PII signal transduction proteins: sensors of alpha-ketoglutarate that regulate nitrogen metabolism. Curr Opin Microbiol. 2005;8:168–173. - PubMed
-
- Ninfa A.J., Atkinson M.R. PII signal transduction proteins. Trends Microbiol. 2000;8:172–179. - PubMed
-
- Leigh J.A., Dodsworth J.A. Nitrogen regulation in bacteria and archaea. Annu Rev Microbiol. 2007;61:349–377. - PubMed
-
- MacPherson K.H., Xu Y., Cheah E., Carr P.D., van Heeswijk W.C., Westerhoff H.V., Luque E., Vasudevan S.G., Ollis D.L. Crystallization and preliminary X-ray analysis of Escherichia coli GlnK. Acta Crystallogr D Biol Crystallogr. 1998;54:996–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

