Spatial regulation of VEGF receptor endocytosis in angiogenesis
- PMID: 23354168
- PMCID: PMC3901019
- DOI: 10.1038/ncb2679
Spatial regulation of VEGF receptor endocytosis in angiogenesis
Abstract
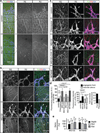
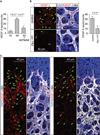
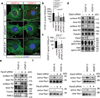
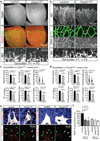
Activities as diverse as migration, proliferation and patterning occur simultaneously and in a coordinated fashion during tissue morphogenesis. In the growing vasculature, the formation of motile, invasive and filopodia-carrying endothelial sprouts is balanced with the stabilization of blood-transporting vessels. Here, we show that sprouting endothelial cells in the retina have high rates of VEGF uptake, VEGF receptor endocytosis and turnover. These internalization processes are opposed by atypical protein kinase C activity in more stable and mature vessels. aPKC phosphorylates Dab2, a clathrin-associated sorting protein that, together with the transmembrane protein ephrin-B2 and the cell polarity regulator PAR-3, enables VEGF receptor endocytosis and downstream signal transduction. Accordingly, VEGF receptor internalization and the angiogenic growth of vascular beds are defective in loss-of-function mice lacking key components of this regulatory pathway. Our work uncovers how vessel growth is dynamically controlled by local VEGF receptor endocytosis and the activity of cell polarity proteins.
Figures

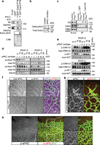
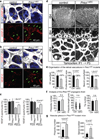
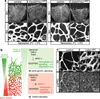
Comment in
-
Development: growing a blood vessel network.Nat Rev Mol Cell Biol. 2013 Mar;14(3):127. doi: 10.1038/nrm3533. Epub 2013 Feb 13. Nat Rev Mol Cell Biol. 2013. PMID: 23403720 No abstract available.
-
Endocytosis regulates VEGF signalling during angiogenesis.Nat Cell Biol. 2013 Mar;15(3):233-5. doi: 10.1038/ncb2705. Nat Cell Biol. 2013. PMID: 23449144
References
-
- Palamidessi A, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. - PubMed
-
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 2005;6:112–126. - PubMed
-
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7:359–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

