S-nitrosoglutathione reductase deficiency increases mutagenesis from alkylation in mouse liver
- PMID: 23354311
- PMCID: PMC3643423
- DOI: 10.1093/carcin/bgt031
S-nitrosoglutathione reductase deficiency increases mutagenesis from alkylation in mouse liver
Abstract
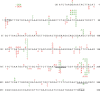
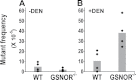
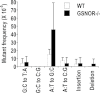
In human hepatocellular carcinoma (HCC) and many other cancers, somatic point mutations are highly prevalent, yet the mechanisms critical in their generation remain poorly understood. S-nitrosoglutathione reductase (GSNOR), a key regulator of protein S-nitrosylation, is frequently deficient in human HCC. Targeted deletion of the GSNOR gene in mice can reduce the activity of the DNA repair protein O (6)-alkylguanine-DNA alkyltransferase (AGT) and promote both carcinogen-induced and spontaneous HCC. In this study, we report that following exposure to the environmental carcinogen diethylnitrosamine, the mutation frequency of a transgenic reporter in the liver of GSNOR-deficient mice (GSNOR(-/-)) is significantly higher than that in wild-type control. In wild-type mice, diethylnitrosamine treatment does not significantly increase the frequency of the transition from G:C to A:T, a mutation deriving from diethylnitrosamine-induced O (6)-ethylguanines that are normally repaired by AGT. In contrast, the frequency of this transition from diethylnitrosamine is increased ~20 times in GSNOR(-/-) mice. GSNOR deficiency also significantly increases the frequency of the transversion from A:T to T:A, a mutation not affected by AGT. GSNOR deficiency in our experiments does not significantly affect either the frequencies of the other diethylnitrosamine-induced point mutations or hepatocyte proliferation. Thus, GSNOR deficiency, through both AGT-dependent and AGT-independent pathways, significantly raises the rates of specific types of DNA mutations. Our results demonstrate a critical role for GSNOR in maintaining genomic integrity in mice and support the hypothesis that GSNOR deficiency is an important cause of the widespread mutations in human HCC.
Figures


References
-
- Totoki Y., et al. (2011). High-resolution characterization of a hepatocellular carcinoma genome. Nat. Genet., 43, 464–469 - PubMed
-
- Sjöblom T., et al. (2006). The consensus coding sequences of human breast and colorectal cancers. Science, 314, 268–274 - PubMed
-
- Perz J.F., et al. (2006). The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol., 45, 529–538 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

