Design of a cluster-randomized controlled trial of a diabetes prevention program within African-American churches: The Fit Body and Soul study
- PMID: 23354313
- PMCID: PMC3594654
- DOI: 10.1016/j.cct.2013.01.002
Design of a cluster-randomized controlled trial of a diabetes prevention program within African-American churches: The Fit Body and Soul study
Abstract
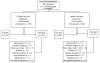
Evidence from varied community settings has shown that the Group Lifestyle Balance (GLB) Program and other adaptations of the Diabetes Prevention Program (DPP) intervention are effective in lowering diabetes risk. Most DPP data originated from studies of pre-diabetic whites, with only sparse evidence of the effect of DPP in African Americans (AAs) in community settings. This paper describes the design, methods, baseline characteristics and cost effective measures, of a single-blinded, cluster-randomized trial of a faith-based adaptation of the GLB program, Fit Body and Soul (FBAS). The major aims are to test efficacy and cost utility of FBAS in twenty AA churches. Randomization occurred at the church level and 604 AA overweight/obese (BMI≥25kg/m(2)) adults with fasting plasma glucose range from normal to pre-diabetic received either FBAS or a health-education comparison program. FBAS is a group-based, multi-level intervention delivered by trained church health advisors (health professionals from within the church), with the goal of ≥7% weight loss, achieved through increasing physical activity, healthy eating and behavior modification. The primary outcome is weight change at 12weeks post intervention. Secondary outcomes include hemoglobin A1C, fasting plasma glucose, waist circumference, blood pressure, physical activity level, quality of life measures, and cost-effectiveness. FBAS is the largest known cohort of AAs enrolled in a faith-based DPP translation. Reliance on health professionals from within the church for program implementation and the cost analysis are unique aspects of this trial. The design provides a model for faith-based DPPs and holds promise for program sustainability and widespread dissemination.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services; Atlanta, GA: Centers for Disease Control and Prevention;
-
- Mokdad A, Ford ES, Bowman, Deitz WH, Vinicor F, Bales BS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors 2001. JAMA. 2003;289(1):76–79. - PubMed
-
- Ackermann RT, Marrero DG. Adapting the diabetes prevention program lifestyle intervention for delivery in the community: The YMCA model. Diabetes Edu. 2008;33(69):69–78. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical