Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids
- PMID: 23354329
- PMCID: PMC4072656
- DOI: 10.1038/nn.3321
Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids
Abstract
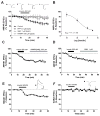
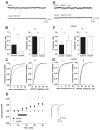
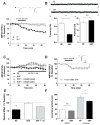
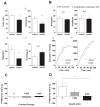
The prevalence of obesity has markedly increased over the past few decades. Exploration of how hunger and satiety signals influence the reward system can help us understand non-homeostatic feeding. Insulin may act in the ventral tegmental area (VTA), a critical site for reward-seeking behavior, to suppress feeding. However, the neural mechanisms underlying insulin effects in the VTA remain unknown. We demonstrate that insulin, a circulating catabolic peptide that inhibits feeding, can induce long-term depression (LTD) of mouse excitatory synapses onto VTA dopamine neurons. This effect requires endocannabinoid-mediated presynaptic inhibition of glutamate release. Furthermore, after a sweetened high-fat meal, which elevates endogenous insulin, insulin-induced LTD is occluded. Finally, insulin in the VTA reduces food anticipatory behavior in mice and conditioned place preference for food in rats. Taken together, these results suggest that insulin in the VTA suppresses excitatory synaptic transmission and reduces anticipatory activity and preference for food-related cues.
Figures



Comment in
-
Insulin and ventral tegmental dopamine: what's impaired and what's intact?Cell Metab. 2013 Apr 2;17(4):469-70. doi: 10.1016/j.cmet.2013.03.011. Cell Metab. 2013. PMID: 23562071
Similar articles
-
Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes.Prog Neuropsychopharmacol Biol Psychiatry. 2014 Jul 3;52:24-7. doi: 10.1016/j.pnpbp.2014.01.019. Epub 2014 Feb 2. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24495779 Free PMC article. Review.
-
Prenatal Ethanol Exposure Persistently Alters Endocannabinoid Signaling and Endocannabinoid-Mediated Excitatory Synaptic Plasticity in Ventral Tegmental Area Dopamine Neurons.J Neurosci. 2017 Jun 14;37(24):5798-5808. doi: 10.1523/JNEUROSCI.3894-16.2017. Epub 2017 May 5. J Neurosci. 2017. PMID: 28476947 Free PMC article.
-
The Epac-Phospholipase Cε Pathway Regulates Endocannabinoid Signaling and Cocaine-Induced Disinhibition of Ventral Tegmental Area Dopamine Neurons.J Neurosci. 2017 Mar 15;37(11):3030-3044. doi: 10.1523/JNEUROSCI.2810-16.2017. Epub 2017 Feb 16. J Neurosci. 2017. PMID: 28209735 Free PMC article.
-
Presynaptic leptin action suppresses excitatory synaptic transmission onto ventral tegmental area dopamine neurons.Biol Psychiatry. 2013 May 1;73(9):860-8. doi: 10.1016/j.biopsych.2012.10.026. Epub 2013 Jan 7. Biol Psychiatry. 2013. PMID: 23305991
-
Synaptic and intrinsic plasticity in the ventral tegmental area after chronic cocaine.Curr Opin Neurobiol. 2019 Feb;54:66-72. doi: 10.1016/j.conb.2018.08.013. Epub 2018 Sep 17. Curr Opin Neurobiol. 2019. PMID: 30237117 Free PMC article. Review.
Cited by
-
Central GLP-1 receptors: Novel molecular targets for cocaine use disorder.Physiol Behav. 2019 Jul 1;206:93-105. doi: 10.1016/j.physbeh.2019.03.026. Epub 2019 Mar 28. Physiol Behav. 2019. PMID: 30930091 Free PMC article. Review.
-
Molecular Mechanisms of Hypothalamic Insulin Resistance.Int J Mol Sci. 2019 Mar 15;20(6):1317. doi: 10.3390/ijms20061317. Int J Mol Sci. 2019. PMID: 30875909 Free PMC article. Review.
-
Junk food diet-induced obesity increases D2 receptor autoinhibition in the ventral tegmental area and reduces ethanol drinking.PLoS One. 2017 Aug 31;12(8):e0183685. doi: 10.1371/journal.pone.0183685. eCollection 2017. PLoS One. 2017. PMID: 28859110 Free PMC article.
-
A high-protein diet for reducing body fat: mechanisms and possible caveats.Nutr Metab (Lond). 2014 Nov 19;11(1):53. doi: 10.1186/1743-7075-11-53. eCollection 2014. Nutr Metab (Lond). 2014. PMID: 25489333 Free PMC article. Review.
-
Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes.Prog Neuropsychopharmacol Biol Psychiatry. 2014 Jul 3;52:24-7. doi: 10.1016/j.pnpbp.2014.01.019. Epub 2014 Feb 2. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24495779 Free PMC article. Review.
References
-
- Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol. 2008;585:38–49. - PubMed
-
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. - PubMed
-
- Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport. 1999;10:221–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

