Effects of a defective endoplasmic reticulum-associated degradation pathway on the stress response, virulence, and antifungal drug susceptibility of the mold pathogen Aspergillus fumigatus
- PMID: 23355008
- PMCID: PMC3623444
- DOI: 10.1128/EC.00319-12
Effects of a defective endoplasmic reticulum-associated degradation pathway on the stress response, virulence, and antifungal drug susceptibility of the mold pathogen Aspergillus fumigatus
Abstract
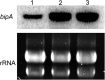
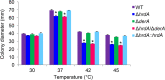
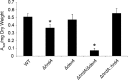
Proteins that are destined for release outside the eukaryotic cell, insertion into the plasma membrane, or delivery to intracellular organelles are processed and folded in the endoplasmic reticulum (ER). An imbalance between the level of nascent proteins entering the ER and the organelle's ability to manage that load results in the accumulation of unfolded proteins. Terminally unfolded proteins are disposed of by ER-associated degradation (ERAD), a pathway that transports the aberrant proteins across the ER membrane into the cytosol for proteasomal degradation. The ERAD pathway was targeted in the mold pathogen Aspergillus fumigatus by deleting the hrdA gene, encoding the A. fumigatus ortholog of Hrd1, the E3 ubiquitin ligase previously shown to contribute to ERAD in other species. Loss of HrdA was associated with impaired degradation of a folding-defective ERAD substrate, CPY*, as well as activation of the unfolded-protein response (UPR). The ΔhrdA mutant showed resistance to voriconazole and reduced thermotolerance but was otherwise unaffected by a variety of environmental stressors. A double-deletion mutant deficient in both HrdA and another component of the same ERAD complex, DerA, was defective in secretion and showed hypersensitivity to ER, thermal, and cell wall stress. However, the ΔhrdA ΔderA mutant remained virulent in mouse and insect infection models. These data demonstrate that HrdA and DerA support complementary ERAD functions that promote survival under conditions of ER stress but are dispensable for virulence in the host environment.
Figures







Similar articles
-
The fungal UPR: a regulatory hub for virulence traits in the mold pathogen Aspergillus fumigatus.Virulence. 2014 Feb 15;5(2):334-40. doi: 10.4161/viru.26571. Epub 2013 Oct 18. Virulence. 2014. PMID: 24189125 Free PMC article. Review.
-
The virulence of the opportunistic fungal pathogen Aspergillus fumigatus requires cooperation between the endoplasmic reticulum-associated degradation pathway (ERAD) and the unfolded protein response (UPR).Virulence. 2011 Jan-Feb;2(1):12-21. doi: 10.4161/viru.2.1.13345. Epub 2011 Jan 1. Virulence. 2011. PMID: 21217201 Free PMC article.
-
Pleiotropic Effects of the P5-Type ATPase SpfA on Stress Response Networks Contribute to Virulence in the Pathogenic Mold Aspergillus fumigatus.mBio. 2021 Oct 26;12(5):e0273521. doi: 10.1128/mBio.02735-21. Epub 2021 Oct 19. mBio. 2021. PMID: 34663092 Free PMC article.
-
Functional Coupling between the Unfolded Protein Response and Endoplasmic Reticulum/Golgi Ca2+-ATPases Promotes Stress Tolerance, Cell Wall Biosynthesis, and Virulence of Aspergillus fumigatus.mBio. 2020 Jun 2;11(3):e01060-20. doi: 10.1128/mBio.01060-20. mBio. 2020. PMID: 32487759 Free PMC article.
-
Secretion stress and antifungal resistance: an Achilles' heel of Aspergillus fumigatus?Med Mycol. 2011 Apr;49 Suppl 1(Suppl 1):S101-6. doi: 10.3109/13693786.2010.497504. Epub 2010 Jul 7. Med Mycol. 2011. PMID: 20608779 Free PMC article. Review.
Cited by
-
Functional exploration of co-expression networks identifies a nexus for modulating protein and citric acid titres in Aspergillus niger submerged culture.Fungal Biol Biotechnol. 2019 Nov 9;6:18. doi: 10.1186/s40694-019-0081-x. eCollection 2019. Fungal Biol Biotechnol. 2019. PMID: 31728200 Free PMC article.
-
The fungal UPR: a regulatory hub for virulence traits in the mold pathogen Aspergillus fumigatus.Virulence. 2014 Feb 15;5(2):334-40. doi: 10.4161/viru.26571. Epub 2013 Oct 18. Virulence. 2014. PMID: 24189125 Free PMC article. Review.
-
The transcription factor CfHac1 regulates the degradation of ubiquitin-mediated ER-associated misfolded proteins and pathogenicity in Colletotrichum fructicola.Stress Biol. 2025 Jun 12;5(1):41. doi: 10.1007/s44154-025-00237-6. Stress Biol. 2025. PMID: 40504284 Free PMC article.
-
Cell death induction in Aspergillus fumigatus: accentuating drug toxicity through inhibition of the unfolded protein response (UPR).Curr Res Microb Sci. 2022 Feb 18;3:100119. doi: 10.1016/j.crmicr.2022.100119. eCollection 2022. Curr Res Microb Sci. 2022. PMID: 35909601 Free PMC article. Review.
-
Autophagy is dispensable to overcome ER stress in the filamentous fungus Aspergillus niger.Microbiologyopen. 2016 Aug;5(4):647-58. doi: 10.1002/mbo3.359. Epub 2016 Mar 29. Microbiologyopen. 2016. PMID: 27027276 Free PMC article.
References
-
- Segal BH. 2009. Aspergillosis. N. Engl. J. Med. 360:1870–1884 - PubMed
-
- Marr KA. 2010. Fungal infections in oncology patients: update on epidemiology, prevention, and treatment. Curr. Opin. Oncol. 22:138–142 - PubMed
-
- Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 65:453–464 - PubMed
-
- Erjavec Z, Kluin-Nelemans H, Verweij PE. 2009. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 15:625–633 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

