Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury
- PMID: 23355340
- PMCID: PMC3625892
- DOI: 10.1152/ajpheart.00883.2012
Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury
Abstract
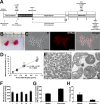
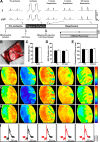
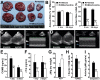
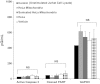
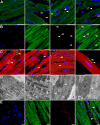
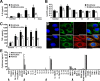
Mitochondrial damage and dysfunction occur during ischemia and modulate cardiac function and cell survival significantly during reperfusion. We hypothesized that transplantation of autologously derived mitochondria immediately prior to reperfusion would ameliorate these effects. New Zealand White rabbits were used for regional ischemia (RI), which was achieved by temporarily snaring the left anterior descending artery for 30 min. Following 29 min of RI, autologously derived mitochondria (RI-mitochondria; 9.7 ± 1.7 × 10(6)/ml) or vehicle alone (RI-vehicle) were injected directly into the RI zone, and the hearts were allowed to recover for 4 wk. Mitochondrial transplantation decreased (P < 0.05) creatine kinase MB, cardiac troponin-I, and apoptosis significantly in the RI zone. Infarct size following 4 wk of recovery was decreased significantly in RI-mitochondria (7.9 ± 2.9%) compared with RI-vehicle (34.2 ± 3.3%, P < 0.05). Serial echocardiograms showed that RI-mitochondria hearts returned to normal contraction within 10 min after reperfusion was started; however, RI-vehicle hearts showed persistent hypokinesia in the RI zone at 4 wk of recovery. Electrocardiogram and optical mapping studies showed that no arrhythmia was associated with autologously derived mitochondrial transplantation. In vivo and in vitro studies show that the transplanted mitochondria are evident in the interstitial spaces and are internalized by cardiomyocytes 2-8 h after transplantation. The transplanted mitochondria enhanced oxygen consumption, high-energy phosphate synthesis, and the induction of cytokine mediators and proteomic pathways that are important in preserving myocardial energetics, cell viability, and enhanced post-infarct cardiac function. Transplantation of autologously derived mitochondria provides a novel technique to protect the heart from ischemia-reperfusion injury.
Figures


References
-
- Bloch DB, Yu JH, Yang WH, Graeme-Cook F, Lindor KD, Viswanathan A, Bloch KD, Nakajima A. The cytoplasmic dot staining pattern is detected in a subgroup of patients with primary biliary cirrhosis. J Rheumatol 32: 477–483, 2005 - PubMed
-
- Chan Y, Tong HQ, Beggs AH, Kunkel LM. Human skeletal muscle-specific alpha-actinin-2 and -3 isoforms form homodimers and heterodimers in vitro and in vivo. Biochem Biophys Res Commun 248: 134–139, 1998 - PubMed
-
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319: 1405–1412, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

