Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers
- PMID: 23355383
- PMCID: PMC3627940
- DOI: 10.1152/ajplung.00181.2012
Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers
Abstract
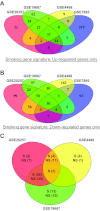
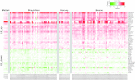
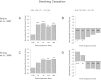
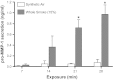
Organotypic culture of human primary bronchial epithelial cells is a useful in vitro system to study normal biological processes and lung disease mechanisms, to develop new therapies, and to assess the biological perturbations induced by environmental pollutants. Herein, we investigate whether the perturbations induced by cigarette smoke (CS) and observed in the epithelium of smokers' airways are reproducible in this in vitro system (AIR-100 tissue), which has been shown to recapitulate most of the characteristics of the human bronchial epithelium. Human AIR-100 tissues were exposed to mainstream CS for 7, 14, 21, or 28 min at the air-liquid interface, and we investigated various biological endpoints [e.g., gene expression and microRNA profiles, matrix metalloproteinase 1 (MMP-1) release] at multiple postexposure time points (0.5, 2, 4, 24, 48 h). By performing a Gene Set Enrichment Analysis, we observed a significant enrichment of human smokers' bronchial epithelium gene signatures derived from different public transcriptomics datasets in CS-exposed AIR-100 tissue. Comparison of in vitro microRNA profiles with microRNA data from healthy smokers highlighted various highly translatable microRNAs associated with inflammation or with cell cycle processes that are known to be perturbed by CS in lung tissue. We also found a dose-dependent increase of MMP-1 release by AIR-100 tissue 48 h after CS exposure in agreement with the known effect of CS on this collagenase expression in smokers' tissues. In conclusion, a similar biological perturbation than the one observed in vivo in smokers' airway epithelium could be induced after a single CS exposure of a human organotypic bronchial epithelium-like tissue culture.
Figures




References
-
- Academies NRCotN Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, D.C.: The National Academies Press, 2007
-
- Balharry D, Sexton K, Berube KA. An in vitro approach to assess the toxicity of inhaled tobacco smoke components: nicotine, cadmium, formaldehyde and urethane. Toxicology 244: 66–76, 2008 - PubMed
-
- Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol 20: 595–604, 1999 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

