Implementation of standardized assessment and reporting of myocardial infarction in contemporary randomized controlled trials: a systematic review
- PMID: 23355654
- PMCID: PMC3604645
- DOI: 10.1093/eurheartj/eht003
Implementation of standardized assessment and reporting of myocardial infarction in contemporary randomized controlled trials: a systematic review
Abstract
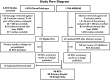
Myocardial infarction (MI) is a key endpoint in randomized controlled trials (RCTs), but heterogeneous definitions limit comparisons across RCTs or meta-analyses. The 2000 European Society of Cardiology/American College of Cardiology MI redefinition and the 2007 universal MI definition consensus documents made recommendations to address this issue. In cardiovascular randomized trials, we evaluated the impact of implementation of three key recommendations from these reports-troponin use to define MI; separate reporting of spontaneous and procedure-related MI; and infarct size reporting. We searched ClinicalTrials.gov and MEDLINE databases for cardiovascular RCTs with more than 500 patients in which enrolment began between September 2000 and July 2012 and that listed MI in the primary endpoint. We searched English-language publications with primary results or design papers. Of 3222 studies screened, 96 (3.0%) met our criteria. We extracted enrolment start date, number of patients and MI events, follow-up duration, and coronary revascularization rate. Data extraction quality was assessed by duplicated extractions. Of 96 RCTs, 80 had a primary results publication, comprising 608 091 patients and 43 621 endpoint MIs. Myocardial infarction represented 45.3% (95% confidence interval, 40.2-50.4) of events in the primary composite endpoint. Troponin defined MI in 57% (53/93) of trials with an MI definition available. Of these RCTs, three used troponin only if creatine kinase-MB was unavailable, six used troponin to define peri-procedural MI, seven specified the 99th percentile as the MI decision limit, and three reported spontaneous and procedure-related MI separately. None reported biomarker-based infarct size, but five reported MI as multiples of the assay upper limit of normal. Although MI is a major component of cardiovascular RCT primary endpoints, standardized MI reporting and implementation of consensus document recommendations for MI definition are limited. Developing appropriate strategies for uniform implementation is required.
Figures

References
-
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction: The Joint European Society of Cardiology/American College of Cardiology Committee. J Am Coll Cardiol. 2000;36:959–969. - PubMed
-
- Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. - PubMed
-
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. - PubMed
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. - PubMed
-
- Guidance on New Law (Public Law 110-85) enacted to expand the scope of ClinicalTrials.gov: registration http://prsinfo.clinicaltrials.gov/fdaaa.html. Accessed 4 May 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

