Current trends in negative immuno-synergy between two sexually transmitted infectious viruses: HIV-1 and HSV-1/2
- PMID: 23355766
- PMCID: PMC3552495
Current trends in negative immuno-synergy between two sexually transmitted infectious viruses: HIV-1 and HSV-1/2
Abstract
In the current era of effective anti-retroviral therapy, immuno-compromised patients with HIV-1 infection do live long enough to suffer diseases caused by many opportunistic infections, such as herpes simplex virus type 1 and/or type 2 (HSV-1/2). An estimated two-third of the 40 million individuals that have contracted HIV-1 worldwide are co-infected with HSV-1/2 viruses, the causative agents of ocular oro-facial and genital herpes. The highest prevalence of HIV and HSV-1/2 infections are confined to the same regions of Sub-Saharan Africa. HSV-1/2 infections affect HIV-1 immunity, and vice versa. While important research gains have been made in understanding herpes and HIV immunity, the cellular and molecular mechanisms underlying the crosstalk between HSV-1/2 and HIV co-infection remain to be fully elucidated. Understanding the mechanisms behind the apparent HSV/HIV negative immuno-synergy maybe the key to successful HSV and HIV vaccines; both are currently unavailable. An effective herpes immunotherapeutic vaccine would in turn - indirectly - contribute in reducing HIV epidemic. The purpose of this review is: (i) to summarize the current trends in understanding the negative immuno-crosstalk between HIV and HSV-1/2 infections; and (ii) to discuss the possibility of developing a novel mucosal herpes immunotherapeutic strategy or even a combined or chimeric immunotherapeutic vaccine that simultaneously targets HIV and HSV-1/2 infections. These new trends in immunology of HSV-1/2 and HIV co-infections should become part of current efforts in preventing sexually transmitted infections. The alternative is needed to balance the ethical and financial concerns associated with the rising number of unsuccessful mono-valent clinical vaccine trials.
Figures
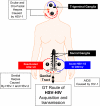
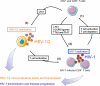



References
-
- Strick LB, Wald A, Celum C. Clin. Infect. Dis. 2006;43:347. - PubMed
