Low cost tuberculosis vaccine antigens in capsules: expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro
- PMID: 23355891
- PMCID: PMC3552857
- DOI: 10.1371/journal.pone.0054708
Low cost tuberculosis vaccine antigens in capsules: expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro
Abstract
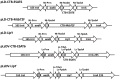
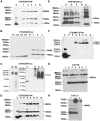
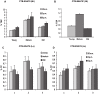
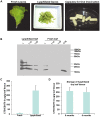
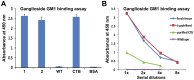
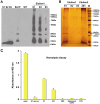
Tuberculosis (TB) caused by Mycobacterium tuberculosis is one of the leading fatal infectious diseases. The development of TB vaccines has been recognized as a major public health priority by the World Health Organization. In this study, three candidate antigens, ESAT-6 (6 kDa early secretory antigenic target) and Mtb72F (a fusion polyprotein from two TB antigens, Mtb32 and Mtb39) fused with cholera toxin B-subunit (CTB) and LipY (a cell wall protein) were expressed in tobacco and/or lettuce chloroplasts to facilitate bioencapsulation/oral delivery. Site-specific transgene integration into the chloroplast genome was confirmed by Southern blot analysis. In transplastomic leaves, CTB fusion proteins existed in soluble monomeric or multimeric forms of expected sizes and their expression levels varied depending upon the developmental stage and time of leaf harvest, with the highest-level of accumulation in mature leaves harvested at 6PM. The CTB-ESAT6 and CTB-Mtb72F expression levels reached up to 7.5% and 1.2% of total soluble protein respectively in mature tobacco leaves. Transplastomic CTB-ESAT6 lettuce plants accumulated up to 0.75% of total leaf protein. Western blot analysis of lyophilized lettuce leaves stored at room temperature for up to six months showed that the CTB-ESAT6 fusion protein was stable and preserved proper folding, disulfide bonds and assembly into pentamers for prolonged periods. Also, antigen concentration per gram of leaf tissue was increased 22 fold after lyophilization. Hemolysis assay with purified CTB-ESAT6 protein showed partial hemolysis of red blood cells and confirmed functionality of the ESAT-6 antigen. GM1-binding assay demonstrated that the CTB-ESAT6 fusion protein formed pentamers to bind with the GM1-ganglioside receptor. The expression of functional Mycobacterium tuberculosis antigens in transplastomic plants should facilitate development of a cost-effective and orally deliverable TB booster vaccine with potential for long-term storage at room temperature. To our knowledge, this is the first report of expression of TB vaccine antigens in chloroplasts.
Conflict of interest statement
Figures

Similar articles
-
Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery.Plant Biotechnol J. 2010 Feb;8(2):223-42. doi: 10.1111/j.1467-7652.2009.00479.x. Epub 2009 Dec 28. Plant Biotechnol J. 2010. PMID: 20051036 Free PMC article.
-
Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts.J Mol Biol. 2001 Aug 31;311(5):1001-9. doi: 10.1006/jmbi.2001.4921. J Mol Biol. 2001. PMID: 11531335 Free PMC article.
-
Synthesis of plant-based, self-adjuvanted, dual antigen specific to Mycobacterium tuberculosis as a novel tuberculosis subunit vaccine that elicits immunogenicity in rabbit.Biotechnol Lett. 2023 Jun;45(5-6):703-717. doi: 10.1007/s10529-023-03371-1. Epub 2023 Apr 19. Biotechnol Lett. 2023. PMID: 37074553 Free PMC article.
-
Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis.Vaccine. 2014 Feb 3;32(6):712-6. doi: 10.1016/j.vaccine.2013.11.065. Epub 2013 Dec 2. Vaccine. 2014. PMID: 24300592 Review.
-
Production of biopharmaceuticals and vaccines in plants via the chloroplast genome.Biotechnol J. 2006 Oct;1(10):1071-9. doi: 10.1002/biot.200600145. Biotechnol J. 2006. PMID: 17004305 Review.
Cited by
-
Potential for Developing Plant-Derived Candidate Vaccines and Biologics against Emerging Coronavirus Infections.Pathogens. 2021 Aug 19;10(8):1051. doi: 10.3390/pathogens10081051. Pathogens. 2021. PMID: 34451516 Free PMC article. Review.
-
Codon Optimization to Enhance Expression Yields Insights into Chloroplast Translation.Plant Physiol. 2016 Sep;172(1):62-77. doi: 10.1104/pp.16.00981. Epub 2016 Jul 27. Plant Physiol. 2016. PMID: 27465114 Free PMC article.
-
Plant-based oral vaccines against zoonotic and non-zoonotic diseases.Plant Biotechnol J. 2016 Nov;14(11):2079-2099. doi: 10.1111/pbi.12604. Epub 2016 Aug 23. Plant Biotechnol J. 2016. PMID: 27442628 Free PMC article. Review.
-
Exploring recent progress of molecular farming for therapeutic and recombinant molecules in plant systems.Heliyon. 2024 Sep 7;10(18):e37634. doi: 10.1016/j.heliyon.2024.e37634. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309966 Free PMC article. Review.
-
Plant Platforms for Efficient Heterologous Protein Production.Biotechnol Bioprocess Eng. 2021;26(4):546-567. doi: 10.1007/s12257-020-0374-1. Epub 2021 Aug 7. Biotechnol Bioprocess Eng. 2021. PMID: 34393545 Free PMC article. Review.
References
-
- WHO (2011) Global Tuberculosis Control. WHO report 2011. World Health Organisation. Available: http://www.who.int/tb/publications/global_report/en/.
-
- Brewer TF, Colditz GA (1995) Relationship between Bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis 20: 126–135. - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, et al. (1994) Efficacy of BCG vaccine in the prevention of Tuberculosis. JAMA 271: 698–702. - PubMed
-
- Xing Z, Lichty BD (2006) Use of recombinant virus-vectored tuberculosis vaccines for respiratory mucosal immunization. Tuberculosis 86: 211–217. - PubMed
-
- Tyagi AK, Nangpal P, Satchidanandam V (2011) Development of vaccines against tuberculosis. Tuberculosis 91: 469–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

