The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496
- PMID: 23356491
- PMCID: PMC3906856
- DOI: 10.1111/bjh.12222
The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496
Abstract
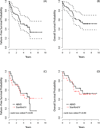
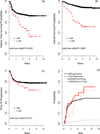
There is a lack of contemporary prospective data examining the adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) and Stanford V (SV; doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone) regimens in older Hodgkin lymphoma (HL) patients. Forty-four advanced-stage, older HL patients (aged ≥60 years) were treated on the randomized study, E2496. Toxicities were mostly similar between chemotherapy regimens, although 24% of older patients developed bleomycin lung toxicity (BLT), which occurred mainly with ABVD (91%). Further, the BLT-related mortality rate was 18%. The overall treatment-related mortality for older HL patients was 9% vs. 0·3% for patients aged <60 years (P < 0·001). Among older patients, there were no survival differences between ABVD and SV. According to age, outcomes were significantly inferior for older versus younger patients (5-year failure-free survival: 48% vs. 74%, respectively, P = 0·002; 5-year overall survival: 58% and 90%, respectively, P < 0·0001), although time-to-progression (TTP) was not significantly different (5-year TTP: 68% vs. 78%, respectively, P = 0·37). Furthermore, considering progression and death without progression as competing risks, the risk of progression was not different between older and younger HL patients (5 years: 30% and 23%, respectively, P = 0·30); however, the incidence of death without progression was significantly increased for older HL patients (22% vs. 9%, respectively, P < 0·0001). Altogether, the marked HL age-dependent survival differences appeared attributable primarily to non-HL events.
Trial registration: ClinicalTrials.gov NCT00003389.
© 2013 Blackwell Publishing Ltd.
Conflict of interest statement
Figures

References
-
- Azambuja E, Fleck JF, Batista RG, Menna Barreto SS. Bleomycin lung toxicity: who are the patients with increased risk? Pulm Pharmacol Ther. 2005;18:363–366. - PubMed
-
- Ballova V, Ruffer JU, Haverkamp H, Pfistner B, Muller-Hermelink HK, Duhmke E, Worst P, Wilhelmy M, Naumann R, Hentrich M, Eich HT, Josting A, Loffler M, Diehl V, Engert A. A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin's disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly) Ann Oncol. 2005;16:124–131. - PubMed
-
- Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood. 2008;111:2977–2983. - PubMed
-
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. - PubMed
-
- Enblad G, Glimelius B, Sundstrom C. Treatment outcome in Hodgkin's disease in patients above the age of 60: a population-based study. Ann Oncol. 1991;2:297–302. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- CA21076/CA/NCI NIH HHS/United States
- U10 CA013650/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- CA13650/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- CA77440/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- CA77202/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

