Thiol/disulfide redox states in signaling and sensing
- PMID: 23356510
- PMCID: PMC4323379
- DOI: 10.3109/10409238.2013.764840
Thiol/disulfide redox states in signaling and sensing
Abstract
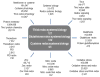
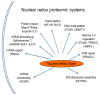
Rapid advances in redox systems biology are creating new opportunities to understand complexities of human disease and contributions of environmental exposures. New understanding of thiol-disulfide systems have occurred during the past decade as a consequence of the discoveries that thiol and disulfide systems are maintained in kinetically controlled steady states displaced from thermodynamic equilibrium, that a widely distributed family of NADPH oxidases produces oxidants that function in cell signaling and that a family of peroxiredoxins utilize thioredoxin as a reductant to complement the well-studied glutathione antioxidant system for peroxide elimination and redox regulation. This review focuses on thiol/disulfide redox state in biologic systems and the knowledge base available to support development of integrated redox systems biology models to better understand the function and dysfunction of thiol-disulfide redox systems. In particular, central principles have emerged concerning redox compartmentalization and utility of thiol/disulfide redox measures as indicators of physiologic function. Advances in redox proteomics show that, in addition to functioning in protein active sites and cell signaling, cysteine residues also serve as redox sensors to integrate biologic functions. These advances provide a framework for translation of redox systems biology concepts to practical use in understanding and treating human disease. Biological responses to cadmium, a widespread environmental agent, are used to illustrate the utility of these advances to the understanding of complex pleiotropic toxicities.
Conflict of interest statement
This work was supported by the National Institute of Environmental Health Sciences (ES011195, ES009047). The authors report no conflicts of interest.
Figures



References
-
- Anderson CL, Iyer SS, Ziegler TR, Jones DP. Control of extracellular cysteine/cystine redox state by HT29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
