Clinical response and symptomatic remission in short- and long-term trials of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder
- PMID: 23356790
- PMCID: PMC3568402
- DOI: 10.1186/1471-244X-13-39
Clinical response and symptomatic remission in short- and long-term trials of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder
Abstract
Background: Despite the overall high degree of response to pharmacotherapy, consensus is lacking on how to judge clinical response or define optimal treatment/remission when treating adults with attention-deficit/hyperactivity disorder (ADHD). This study examined clinical response and symptomatic remission in analyses of 2 studies of lisdexamfetamine dimesylate (LDX) in adults with ADHD.
Methods: In a 4-week, double-blind, forced-dose trial, adults with ADHD were randomized to LDX 30, 50, and 70 mg/day (mg/d) or placebo. In a second, open-label, follow-up trial, adults entering from the 4-week study were titrated to an "optimal" LDX dose (30 mg/d [n=44], 50 mg/d [n=112], and 70 mg/d [n=171]) over 4 weeks, and maintained for 11 additional months. The ADHD Rating Scale IV (ADHD-RS-IV) with adult prompts and the Clinical Global Impressions-Improvement (CGI-I) scale assessed efficacy. Clinical response was defined, post hoc, as ≥30% reduction from baseline in ADHD-RS-IV and CGI-I rating of 1 or 2; symptomatic remission was defined as ADHD-RS-IV total score ≤18. Log rank analysis examined overall significance among the treatment groups in time to response or remission.
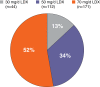
Results: Four hundred and fourteen participants in the 4-week study and 345 in the open-label, extension study were included in the efficacy populations. All LDX groups improved by ADHD-RS-IV and CGI-I scores in both studies. In the 4-week study (n=414), 69.3% responded and 45.5% achieved remission with LDX (all doses); 37.1% responded and 16.1% achieved remission with placebo; time (95% CI) to median clinical response (all LDX doses) was 15.0 (15.0, 17.0) days and to remission was 31.0 (28.0, 37.0) days (P<.0001 overall). In the open-label study, with LDX (all doses), 313 (95.7%) and 278 (85.0%) of 327 participants with evaluable maintenance-phase data met criteria for response and remission, respectively. Of participants who completed dose optimization, 75.2% remained responders and 65.7% remained in remission in the 12-month study. Overall, 285 (82.6%) and 227 (65.8%) of 345 participants were responders and remitters, respectively, at their final visits.
Conclusion: In the long-term study, with open-label, dose-optimized LDX treatment, most adults with ADHD achieved clinical response and/or symptomatic remission; almost two-thirds maintained symptomatic remission over the remaining 11 months.
Trial registration: Clinical Trial Numbers: NCT00334880 and NCT01070394CLINICAL TRIAL REGISTRY: clinicaltrials.gov.
Figures



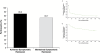
References
-
- Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child Adolesc Psychiatr Clin N Am. 2000;9(3):541–555. vii. - PubMed
-
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K. et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. - DOI - PMC - PubMed
-
- Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Harpold T, Dunkel S, Dougherty M, Aleardi M, Spencer T. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59(9):829–835. doi: 10.1016/j.biopsych.2005.09.011. - DOI - PubMed
-
- Jain U, Hechtman L, Weiss M, Ahmed TS, Reiz JL, Donnelly GA, Harsanyi Z, Darke AC. Efficacy of a novel biphasic controlled-release methylphenidate formula in adults with attention-deficit/hyperactivity disorder: results of a double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2007;68(2):268–277. doi: 10.4088/JCP.v68n0213. - DOI - PubMed
-
- Adler LA, Goodman DW, Kollins SH, Weisler RH, Krishnan S, Zhang Y, Biederman J. on behalf of the 303 study group. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–1373. doi: 10.4088/JCP.v69n0903. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

