Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature
- PMID: 23357369
- PMCID: PMC3581686
- DOI: 10.1016/j.biomaterials.2013.01.017
Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature
Abstract
Microscale and nanoscale structures can spatially pattern endothelial cells (ECs) into parallel-aligned organization, mimicking their cellular alignment in blood vessels exposed to laminar shear stress. However, the effects of spatial patterning on the function and global transcriptome of ECs are incompletely characterized. We used both parallel-aligned micropatterned and nanopatterned biomaterials to evaluate the effects of spatial patterning on the phenotype of ECs, based on gene expression profiling, functional characterization of monocyte adhesion, and quantification of cellular morphology. We demonstrate that both micropatterned and aligned nanofibrillar biomaterials could effectively guide EC organization along the direction of the micropatterned channels or nanofibrils, respectively. The ability of ECs to sense spatial patterning cues were abrogated in the presence of cytoskeletal disruption agents. Moreover, both micropatterned and aligned nanofibrillar substrates promoted an athero-resistant EC phenotype by reducing endothelial adhesiveness for monocytes and platelets, as well as by downregulating the expression of adhesion proteins and chemokines. We further found that micropatterned ECs have a transcriptional signature that is unique from non-patterned ECs, as well as from ECs aligned by shear stress. These findings highlight the importance of spatial patterning cues in guiding EC organization and function, which may have clinical relevance in the development of vascular grafts that promote patency.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
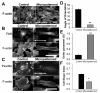


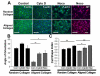

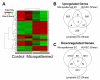
Similar articles
-
Nanoscale Patterning of Extracellular Matrix Alters Endothelial Function under Shear Stress.Nano Lett. 2016 Jan 13;16(1):410-9. doi: 10.1021/acs.nanolett.5b04028. Epub 2015 Dec 28. Nano Lett. 2016. PMID: 26670737 Free PMC article.
-
The modulation of endothelial cell morphology, function, and survival using anisotropic nanofibrillar collagen scaffolds.Biomaterials. 2013 May;34(16):4038-4047. doi: 10.1016/j.biomaterials.2013.02.036. Epub 2013 Mar 5. Biomaterials. 2013. PMID: 23480958 Free PMC article.
-
Differential effects of orbital and laminar shear stress on endothelial cells.J Vasc Surg. 2005 May;41(5):869-80. doi: 10.1016/j.jvs.2005.01.020. J Vasc Surg. 2005. PMID: 15886673
-
Micropatterned coculture of vascular endothelial and smooth muscle cells on layered electrospun fibrous mats toward blood vessel engineering.J Biomed Mater Res A. 2015 Jun;103(6):1949-60. doi: 10.1002/jbm.a.35332. Epub 2014 Sep 17. J Biomed Mater Res A. 2015. PMID: 25204306
-
Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review.Histol Histopathol. 2004 Apr;19(2):535-64. doi: 10.14670/HH-19.535. Histol Histopathol. 2004. PMID: 15024715 Review.
Cited by
-
Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering.Adv Healthc Mater. 2014 May;3(5):628-41. doi: 10.1002/adhm.201300620. Epub 2014 Jan 20. Adv Healthc Mater. 2014. PMID: 24443420 Free PMC article. Review.
-
Bioengineering Cell Therapy for Treatment of Peripheral Artery Disease.Arterioscler Thromb Vasc Biol. 2024 Mar;44(3):e66-e81. doi: 10.1161/ATVBAHA.123.318126. Epub 2024 Jan 4. Arterioscler Thromb Vasc Biol. 2024. PMID: 38174560 Free PMC article. Review.
-
The extracellular fluid macromolecular composition differentially affects cell-substrate adhesion and cell morphology.Sci Rep. 2019 Jun 11;9(1):8505. doi: 10.1038/s41598-019-44960-3. Sci Rep. 2019. PMID: 31186501 Free PMC article.
-
The path to a hemocompatible cardiovascular implant: Advances and challenges of current endothelialization strategies.Front Cardiovasc Med. 2022 Sep 14;9:971028. doi: 10.3389/fcvm.2022.971028. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36186971 Free PMC article. Review.
-
Enhancing Stent Effectiveness with Nanofeatures.Methodist Debakey Cardiovasc J. 2016 Sep;12(3):163-168. doi: 10.14797/mdcj-12-3-163. Methodist Debakey Cardiovasc J. 2016. PMID: 27826371 Free PMC article. Review.
References
-
- Vartanian KB, Berny MA, McCarty OJT, Hanson SR, Hinds MT. Cytoskeletal structure regulates endothelial cell immunogenicity independent of fluid shear stress. Am J Physiol Cell Physiol. 2010;298(2):C333–C341. - PubMed
-
- Businaro R, Tagliani A, Buttari B, Profumo E, Ippoliti F, Di Cristofano C, et al. Cellular and molecular players in the atherosclerotic plaque progression. Ann N Y Acad Sci. 2012:1262134–141. - PubMed
-
- Gerszten RE, Lim YC, Ding HT, Snapp K, Kansas G, Dichek DA, et al. Adhesion of monocytes to vascular cell adhesion molecule-1-transduced human endothelial cells: Implications for atherogenesis. Circ Res. 1998;82(8):871–878. - PubMed
-
- Gawaz M, Neumann FJ, Dickfeld T, Koch W, Laugwitz KL, Adelsberger H, et al. Activated platelets induce monocyte chemotactic protein-1 secretion and surface expression of intercellular adhesion molecule-1 on endothelial cells. Circulation. 1998;98(12):1164–1171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

