CD11a regulates effector CD8 T cell differentiation and central memory development in response to infection with Listeria monocytogenes
- PMID: 23357382
- PMCID: PMC3639604
- DOI: 10.1128/IAI.00749-12
CD11a regulates effector CD8 T cell differentiation and central memory development in response to infection with Listeria monocytogenes
Abstract
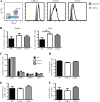
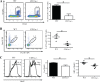
β2 (CD18) integrins with α-chains CD11a, -b, -c, and -d are important adhesion molecules necessary for leukocyte migration and cellular interactions. CD18 deficiency leads to recurrent bacterial infections and poor wound healing due to reduced migration of leukocytes to inflammatory sites. CD8 T cells also upregulate CD11a, CD11b, and CD11c upon activation. However, the role these molecules play for CD8 T cells in vivo is not known. To determine the function of individual β2 integrins, we examined CD8 T cell responses to Listeria monocytogenes infection in CD11a-, CD11b-, and CD11c-deficient mice. The absence of CD11b or CD11c had no effect on the generation of antigen-specific CD8 T cells. In contrast, the magnitude of the primary CD8 T cell response in CD11a-deficient mice was significantly reduced. Moreover, the response in CD11a(-/-) mice exhibited reduced differentiation of short-lived effector cells (KLRG1(hi) CD127(lo)), although cytokine and granzyme B production levels were unaffected. Notably, CD11a deficiency resulted in greatly enhanced generation of CD62L(+) central memory cells. Surprisingly, CD8 T cells lacking CD11a mounted a robust secondary response to infection. Taken together, these findings demonstrated that CD11a expression contributes to expansion and differentiation of primary CD8 T cells but may be dispensable for secondary responses to infection.
Figures





References
-
- McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. 1992. CD11b(Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J. Immunol. 149:1326–1333 - PubMed
-
- Huleatt JW, Lefrançois L. 1995. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8(+) T cells in vivo. J. Immunol. 154:5684–5693 - PubMed
-
- Hynes RO. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25 - PubMed
-
- Anderson DC, Springer TA. 1987. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 38:175–194 - PubMed
-
- Hogg N, Bates PA. 2000. Genetic analysis of integrin function in man: LAD-1 and other syndromes. Matrix Biol. 19:211–222 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

