Pathology and pathophysiology of inhalational anthrax in a guinea pig model
- PMID: 23357384
- PMCID: PMC3639603
- DOI: 10.1128/IAI.01289-12
Pathology and pathophysiology of inhalational anthrax in a guinea pig model
Abstract
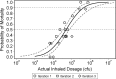
Nonhuman primates (NHPs) and rabbits are the animal models most commonly used to evaluate the efficacy of medical countermeasures against anthrax in support of licensure under the FDA's "Animal Rule." However, a need for an alternative animal model may arise in certain cases. The development of such an alternative model requires a thorough understanding of the course and manifestation of experimental anthrax disease induced under controlled conditions in the proposed animal species. The guinea pig, which has been used extensively for anthrax pathogenesis studies and anthrax vaccine potency testing, is a good candidate for such an alternative model. This study was aimed at determining the median lethal dose (LD50) of the Bacillus anthracis Ames strain in guinea pigs and investigating the natural history, pathophysiology, and pathology of inhalational anthrax in this animal model following nose-only aerosol exposure. The inhaled LD50 of aerosolized Ames strain spores in guinea pigs was determined to be 5.0 × 10(4) spores. Aerosol challenge of guinea pigs resulted in inhalational anthrax with death occurring between 46 and 71 h postchallenge. The first clinical signs appeared as early as 36 h postchallenge. Cardiovascular function declined starting at 20 h postexposure. Hematogenous dissemination of bacteria was observed microscopically in multiple organs and tissues as early as 24 h postchallenge. Other histopathologic findings typical of disseminated anthrax included suppurative (heterophilic) inflammation, edema, fibrin, necrosis, and/or hemorrhage in the spleen, lungs, and regional lymph nodes and lymphocyte depletion and/or lymphocytolysis in the spleen and lymph nodes. This study demonstrated that the course of inhalational anthrax disease and the resulting pathology in guinea pigs are similar to those seen in rabbits and NHPs, as well as in humans.
Figures


Similar articles
-
Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis).Lab Invest. 2003 Aug;83(8):1201-9. doi: 10.1097/01.lab.0000080599.43791.01. Lab Invest. 2003. PMID: 12920249
-
The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation.Arch Pathol Lab Med. 1998 Nov;122(11):982-92. Arch Pathol Lab Med. 1998. PMID: 9822127
-
Inhalational anthrax (Ames aerosol) in naïve and vaccinated New Zealand rabbits: characterizing the spread of bacteria from lung deposition to bacteremia.Front Cell Infect Microbiol. 2012 Jun 28;2:87. doi: 10.3389/fcimb.2012.00087. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919678 Free PMC article.
-
Pathology of inhalational anthrax animal models.Vet Pathol. 2010 Sep;47(5):819-30. doi: 10.1177/0300985810378112. Epub 2010 Jul 23. Vet Pathol. 2010. PMID: 20656900 Review.
-
Animal Models for the Pathogenesis, Treatment, and Prevention of Infection by Bacillus anthracis.Microbiol Spectr. 2015 Feb;3(1):TBS-0001-2012. doi: 10.1128/microbiolspec.TBS-0001-2012. Microbiol Spectr. 2015. PMID: 26104551 Review.
Cited by
-
Dual-Readout Sandwich Immunoassay for Device-Free and Highly Sensitive Anthrax Biomarker Detection.Anal Chem. 2020 Jun 2;92(11):7845-7851. doi: 10.1021/acs.analchem.0c01090. Epub 2020 May 21. Anal Chem. 2020. PMID: 32437125 Free PMC article.
-
Pathology of wild-type and toxin-independent Bacillus anthracis meningitis in rabbits.PLoS One. 2017 Oct 31;12(10):e0186613. doi: 10.1371/journal.pone.0186613. eCollection 2017. PLoS One. 2017. PMID: 29088287 Free PMC article.
-
In vitro exposure system for study of aerosolized influenza virus.Virology. 2017 Jan;500:62-70. doi: 10.1016/j.virol.2016.10.007. Epub 2016 Oct 20. Virology. 2017. PMID: 27771560 Free PMC article.
-
Comprehensive characterization of toxins during progression of inhalation anthrax in a non-human primate model.PLoS Pathog. 2022 Dec 19;18(12):e1010735. doi: 10.1371/journal.ppat.1010735. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36534695 Free PMC article.
-
Current Status and Trends in Prophylaxis and Management of Anthrax Disease.Pathogens. 2020 May 12;9(5):370. doi: 10.3390/pathogens9050370. Pathogens. 2020. PMID: 32408493 Free PMC article. Review.
References
-
- Brachman PS. 1980. Inhalation anthrax. Ann. N. Y. Acad. Sci. 353:83–93 - PubMed
-
- Penn CC, Klotz SA. 1997. Anthrax pneumonia. Semin. Respir. Infect. 12:28–30 - PubMed
-
- Shafazand S, Doyle R, Ruoss S, Weinacker A, Raffin TA. 1999. Inhalational anthrax: epidemiology, diagnosis, and management. Chest 116:1369–1376 - PubMed
-
- Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. 2006. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 144:270–280 - PubMed
-
- Golden HB, Watson LE, Lal H, Verma SK, Foster DM, Kuo SR, Sharma A, Frankel A, Dostal DE. 2009. Anthrax toxin: pathologic effects on the cardiovascular system. Front. Biosci. 14:2335–2357 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

