Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes
- PMID: 23357756
- PMCID: PMC3676863
- DOI: 10.1016/j.mam.2013.01.004
Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes
Abstract
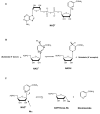
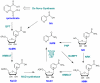
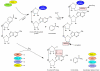
Poly(ADP-ribose) polymerases (PARPs) are NAD(+) dependent enzymes that were identified as DNA repair proteins, however, today it seems clear that PARPs are responsible for a plethora of biological functions. Sirtuins (SIRTs) are NAD(+)-dependent deacetylase enzymes involved in the same biological processes as PARPs raising the question whether PARP and SIRT enzymes may interact with each other in physiological and pathophysiological conditions. Hereby we review the current understanding of the SIRT-PARP interplay in regard to the biochemical nature of the interaction (competition for the common NAD(+) substrate, mutual posttranslational modifications and direct transcriptional effects) and the physiological or pathophysiological consequences of the interactions (metabolic events, oxidative stress response, genomic stability and aging). Finally, we give an overview of the possibilities of pharmacological intervention to modulate PARP and SIRT enzymes either directly, or through modulating NAD(+) homeostasis.
Keywords: Metabolism; Mitochondria; NAD(+); Oxidative stress; Poly(ADP-ribose) polymerase; Sirtuins SIRT1.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
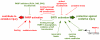



References
-
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. - PubMed
-
- Allinson SL, Dianova, Dianov GL. Poly(ADP-ribose) polymerase in base excision repair: always engaged, but not essential for DNA damage processing. Acta Biochim Pol. 2003;50:169–179. - PubMed
-
- Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54:558–559. - PubMed
-
- Alvarez-Gonzalez R, Mendoza-Alvarez H. Dissection of ADP-ribose polymer synthesis into individual steps of initiation, elongation, and branching. Biochimie. 1995;77:403–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

