Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy
- PMID: 23357769
- PMCID: PMC3623349
- DOI: 10.1128/AAC.02011-12
Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy
Abstract
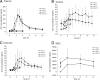
Although azithromycin is extensively used in the treatment of respiratory tract infections as well as skin and skin-related infections, pharmacokinetics of azithromycin in extracellular space fluid of soft tissues, i.e., one of its therapeutic target sites, are not yet fully elucidated. In this study, azithromycin concentration-time profiles in extracellular space of muscle and subcutaneous adipose tissue, but also in plasma and white blood cells, were determined at days 1 and 3 of treatment as well as 2 and 7 days after the end of treatment. Of all compartments, azithromycin concentrations were highest in white blood cells, attesting for intracellular accumulation. However, azithromycin concentrations in both soft tissues were markedly lower than in plasma both during and after treatment. Calculation of the area under the concentration-time curve from 0 to 24 h (AUC(0-24))/MIC(90) ratios for selected pathogens suggests that azithromycin concentrations measured in the present study are subinhibitory at all time points in both soft tissues and at the large majority of observed time points in plasma. Hence, it might be speculated that azithromycin's clinical efficacy relies not only on elevated intracellular concentrations but possibly also on its known pleotropic effects, including immunomodulation and influence on bacterial virulence factors. However, prolonged subinhibitory azithromycin concentrations at the target site, as observed in the present study, might favor the emergence of bacterial resistance and should therefore be considered with concern. In conclusion, this study has added important information to the pharmacokinetic profile of the widely used antibiotic drug azithromycin and evidentiates the need for further research on its potential for induction of bacterial resistance.
Figures
References
-
- Coenen S, Ferech M, Malhotra-Kumar S, Hendrickx E, Suetens C, Goossens H. 2006. European Surveillance of Antimicrobial Consumption (ESAC): outpatient macrolide, lincosamide and streptogramin (MLS) use in Europe. J. Antimicrob. Chemother. 58:418–422 - PubMed
-
- Marra F, Patrick DM, Chong M, Bowie WR. 2006. Antibiotic use among children in British Columbia, Canada. J. Antimicrob. Chemother. 58:830–839 - PubMed
-
- Andes D, Anon J, Jacobs MR, Craig WA. 2004. Application of pharmacokinetics and pharmacodynamics to antimicrobial therapy of respiratory tract infections. Clin. Lab. Med. 24:477–502 - PubMed
-
- Retsema J, Girard A, Schelkly W, Manousos M, Anderson M, Bright G, Borovoy R, Brennan L, Mason R. 1987. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against Gram-negative organisms. Antimicrob. Agents Chemother. 31:1939–1947 - PMC - PubMed
-
- Pichereau S, Moran JJ, Hayney MS, Shukla SK, Sakoulas G, Rose WE. 2012. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J. Antimicrob. Chemother. 67:123–129 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical