Pharmacodynamic variability beyond that explained by MICs
- PMID: 23357773
- PMCID: PMC3623339
- DOI: 10.1128/AAC.01224-12
Pharmacodynamic variability beyond that explained by MICs
Abstract
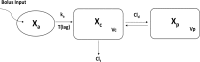
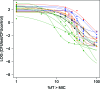
Monte Carlo simulations (MCS) present a powerful tool to evaluate candidate regimens by determining the probability of target attainment. Although these assessments have traditionally incorporated variability in pharmacokinetic (PK) parameters and MICs, consideration of interstrain pharmacodynamic (PD) variability has been neglected. A population PK/PD model was developed for doripenem using murine thigh infection data based on 20 bacterial strains. PK data were fit to a linear two-compartment model with first-order input and elimination processes and an absorption lag time from a separate site (r(2) > 0.96). PK parameters were utilized to simulate free-drug profiles for various regimens in PD studies, from which the percentage of the dosing interval for which free-drug concentrations exceed the MIC of the targeted strain (%fT>MIC) was calculated. Doripenem PD was excellently described with Hill-type models (r(2) > 0.98); significant differences between mean PD estimates determined using a two-stage approach versus population analyses were not observed (P > 0.05); however, the variance in 50% effective concentration (EC50) and maximum effect (Emax) among strains was much greater using the two-stage approach. Even using the population approach, interstrain variability in EC50 (coefficient of variation expressed as a percentage [CV%] = 29.2%) and H (CV% = 46.1%) parameters was substantive, while the variability in Emax (CV% = 19.7%) was modest. This resulted in extensive variability in the range of %fT>MIC targets associated with stasis to those associated with a 2-log10 reduction in bacterial burden (CV% ∼ 50%). It appears that MCS, based on the assumption that PD variability is due to MIC alone, underestimates variability and may consequently underestimate treatment failures.
Figures

References
-
- Ortho-McNeil Pharmaceutical 2007. Doribax package insert. Ortho-McNeil Pharmaceutical, Inc., Raritan, NJ
-
- Livermore DM. 2009. Doripenem: antimicrobial profile and clinical potential. Diagn. Microbiol. Infect. Dis. 63:455–458 - PubMed
-
- AstraZeneca 2007. Merrem package insert. AstraZeneca, Wilmington, DE
-
- Merck & Co 2006. Primaxin IV package insert. Merck & Co., Inc., Whitehouse Station, NJ
-
- Zhanel GG, Ketter N, Rubinstein E, Friedland I, Redman R. 2009. Overview of seizure-inducing potential of doripenem. Drug Saf. 32:709–716 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

