Annexin-phospholipid interactions. Functional implications
- PMID: 23358253
- PMCID: PMC3588008
- DOI: 10.3390/ijms14022652
Annexin-phospholipid interactions. Functional implications
Abstract
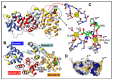
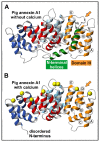
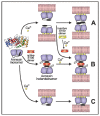
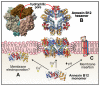
Annexins constitute an evolutionary conserved multigene protein superfamily characterized by their ability to interact with biological membranes in a calcium dependent manner. They are expressed by all living organisms with the exception of certain unicellular organisms. The vertebrate annexin core is composed of four (eight in annexin A6) homologous domains of around 70 amino acids, with the overall shape of a slightly bent ring surrounding a central hydrophilic pore. Calcium- and phospholipid-binding sites are located on the convex side while the N-terminus links domains I and IV on the concave side. The N-terminus region shows great variability in length and amino acid sequence and it greatly influences protein stability and specific functions of annexins. These proteins interact mainly with acidic phospholipids, such as phosphatidylserine, but differences are found regarding their affinity for lipids and calcium requirements for the interaction. Annexins are involved in a wide range of intra- and extracellular biological processes in vitro, most of them directly related with the conserved ability to bind to phospholipid bilayers: membrane trafficking, membrane-cytoskeleton anchorage, ion channel activity and regulation, as well as antiinflammatory and anticoagulant activities. However, the in vivo physiological functions of annexins are just beginning to be established.
Figures

References
-
- Gerke V., Moss S.E. Annexins: From structure to function. Physiol. Rev. 2002;82:331–371. - PubMed
-
- Raynal P., Pollard H.B. Annexins: The problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta. 1994;1197:63–93. - PubMed
-
- Clark G.B., Morgan R.O., Fernandez M.P., Roux S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012;196:695–712. - PubMed
-
- Swairjo M.A., Seaton B.A. Annexin structure and membrane interactions: A molecular perspective. Annu. Rev. Biophys. Biomol. Struct. 1994;23:193–213. - PubMed
-
- Morgan R.O., Jenkins N.A., Gilbert D.J., Copeland N.G., Balsara B.R., Testa J.R., Fernandez M.P. Novel human and mouse annexin A10 are linked to the genome duplications during early chordate evolution. Genomics. 1999;60:40–49. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

