ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines
- PMID: 23358684
- PMCID: PMC4038408
- DOI: 10.1158/0008-5472.CAN-12-3424
ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines
Abstract
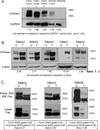
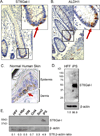
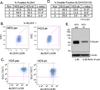
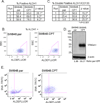
The ST6Gal-I sialyltransferase adds an α2-6-linked sialic acid to the N-glycans of certain receptors. ST6Gal-I mRNA has been reported to be upregulated in human cancer, but a prior lack of antibodies has limited immunochemical analysis of the ST6Gal-I protein. Here, we show upregulated ST6Gal-I protein in several epithelial cancers, including many colon carcinomas. In normal colon, ST6Gal-I localized selectively to the base of crypts, where stem/progenitor cells are found, and the tissue staining patterns were similar to the established stem cell marker ALDH1. Similarly, ST6Gal-I expression was restricted to basal epidermal layers in skin, another stem/progenitor cell compartment. ST6Gal-I was highly expressed in induced pluripotent stem (iPS) cells, with no detectable expression in the fibroblasts from which iPS cells were derived. On the basis of these observations, we investigated further an association of ST6Gal-I with cancer stem cells (CSC). Selection of irinotecan resistance in colon carcinoma cells led to a greater proportion of CSCs compared with parental cells, as measured by the CSC markers CD133 and ALDH1 activity (Aldefluor). These chemoresistant cells exhibited a corresponding upregulation of ST6Gal-I expression. Conversely, short hairpin RNA (shRNA)-mediated attenuation of ST6Gal-I in colon carcinoma cells with elevated endogenous expression decreased the number of CD133/ALDH1-positive cells present in the cell population. Collectively, our results suggest that ST6Gal-I promotes tumorigenesis and may serve as a regulator of the stem cell phenotype in both normal and cancer cell populations.
©2012 AACR.
Conflict of interest statement
Authors have no conflict of interest to disclose.
Figures


References
-
- Dall'Olio F, Chiricolo M. Sialyltransferases in cancer. Glycoconj J. 2001;18:841–850. - PubMed
-
- Zhu Y, Srivatana U, Ullah A, Gagneja H, Berenson CS, Lance P. Suppression of a sialyltransferase by antisense DNA reduces invasiveness of human colon cancer cells in vitro. Biochim Biophys Acta. 2001;1536:148–160. - PubMed
-
- Lin S, Kemmner W, Grigull S, Schlag PM. Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Exp Cell Res. 2002;276:101–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

