Basal stem cells contribute to squamous cell carcinomas in the oral cavity
- PMID: 23358851
- PMCID: PMC3643419
- DOI: 10.1093/carcin/bgt021
Basal stem cells contribute to squamous cell carcinomas in the oral cavity
Abstract
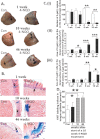
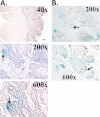
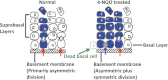
The cells of origin of oral cavity squamous cell carcinoma (OCSCC) are unknown. We used a cell lineage tracing approach (adult K14-CreER(TAM); ROSA26 mice transiently treated with tamoxifen) to identify and track normal epithelial stem cells (SCs) in mouse tongues by X-gal staining and to determine if these cells become neoplastically transformed by treatment with a carcinogen, 4-nitroquinoline 1-oxide (4-NQO). Here, we show that in normal tongue epithelia, X-gal(+) cells formed thin columns throughout the entire epithelium 12 weeks after tamoxifen treatment, indicating that the basal layer contains long-lived SCs that produce progeny by asymmetric division to maintain homeostasis. Carcinogen treatment results in a ~10-fold reduction in the total number of X-gal(+) clonal cell populations and horizontal expansion of X-gal(+) clonal cell columns, a pattern consistent with symmetric division of some SCs. Finally, X-gal(+) SCs are present in papillomas and invasive OCSCCs, and these long-lived X-gal(+) SCs are the cells of origin of these tumors. Moreover, the resulting 4-NQO-induced tumors are multiclonal. These findings provide insights into the identity of the initiating cells of oral cancer.
Figures


References
-
- Siegel R., et al. (2011). Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin., 61, 212–236 - PubMed
-
- Binnie W.H., et al. (1983). Etiology of oral squamous cell carcinoma. J. Oral Pathol., 12, 11–29 - PubMed
-
- Lippman S.M., et al. (2005). Oral cancer prevention and the evolution of molecular-targeted drug development. J. Clin. Oncol., 23, 346–356 - PubMed
-
- Kupferman M.E., et al. (2007). Molecular analysis of anoikis resistance in oral cavity squamous cell carcinoma. Oral Oncol., 43, 440–454 - PubMed
-
- Tsai L.L., et al. (2011). Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med., 40, 621–628 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

