IFITM proteins restrict viral membrane hemifusion
- PMID: 23358889
- PMCID: PMC3554583
- DOI: 10.1371/journal.ppat.1003124
IFITM proteins restrict viral membrane hemifusion
Abstract
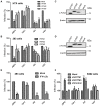
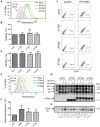
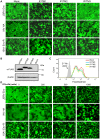
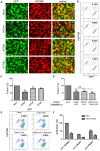
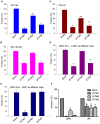
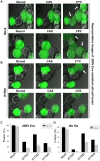
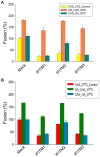
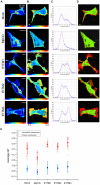
The interferon-inducible transmembrane (IFITM) protein family represents a new class of cellular restriction factors that block early stages of viral replication; the underlying mechanism is currently not known. Here we provide evidence that IFITM proteins restrict membrane fusion induced by representatives of all three classes of viral membrane fusion proteins. IFITM1 profoundly suppressed syncytia formation and cell-cell fusion induced by almost all viral fusion proteins examined; IFITM2 and IFITM3 also strongly inhibited their fusion, with efficiency somewhat dependent on cell types. Furthermore, treatment of cells with IFN also markedly inhibited viral membrane fusion and entry. By using the Jaagsiekte sheep retrovirus envelope and influenza A virus hemagglutinin as models for study, we showed that IFITM-mediated restriction on membrane fusion is not at the steps of receptor- and/or low pH-mediated triggering; instead, the creation of hemifusion was essentially blocked by IFITMs. Chlorpromazine (CPZ), a chemical known to promote the transition from hemifusion to full fusion, was unable to rescue the IFITM-mediated restriction on fusion. In contrast, oleic acid (OA), a lipid analog that generates negative spontaneous curvature and thereby promotes hemifusion, virtually overcame the restriction. To explore the possible effect of IFITM proteins on membrane molecular order and fluidity, we performed fluorescence labeling with Laurdan, in conjunction with two-photon laser scanning and fluorescence-lifetime imaging microscopy (FLIM). We observed that the generalized polarizations (GPs) and fluorescence lifetimes of cell membranes expressing IFITM proteins were greatly enhanced, indicating higher molecularly ordered and less fluidized membranes. Collectively, our data demonstrated that IFITM proteins suppress viral membrane fusion before the creation of hemifusion, and suggested that they may do so by reducing membrane fluidity and conferring a positive spontaneous curvature in the outer leaflets of cell membranes. Our study provides novel insight into the understanding of how IFITM protein family restricts viral membrane fusion and infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Sheehy AM, Gaddis NC, Choi JD, Malim MH (2002) Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418: 646–650. - PubMed
-
- Neil SJ, Zang T, Bieniasz PD (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451: 425–430. - PubMed
-
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, et al. (2004) The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427: 848–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21AI105584/AI/NIAID NIH HHS/United States
- GM066837/GM/NIGMS NIH HHS/United States
- P41 GM1035401/GM/NIGMS NIH HHS/United States
- P41-RRO3155/PHS HHS/United States
- MOP-77649/CAPMC/ CIHR/Canada
- R21 AI105584/AI/NIAID NIH HHS/United States
- P41 RR003155/RR/NCRR NIH HHS/United States
- P50 GM076516/GM/NIGMS NIH HHS/United States
- P41 GM103540/GM/NIGMS NIH HHS/United States
- R21 AG032579/AG/NIA NIH HHS/United States
- R01 GM101539/GM/NIGMS NIH HHS/United States
- P50-GM076516/GM/NIGMS NIH HHS/United States
- R21AG032579/AG/NIA NIH HHS/United States
- R01 GM066837/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

