Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01
- PMID: 23358966
- PMCID: PMC3595424
- DOI: 10.1200/JCO.2012.43.2070
Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01
Abstract
Purpose: We assessed the toxicity and efficacy of dexamethasone and a novel dosing method of Escherichia coli L-asparaginase (EC-Asnase) in children and adolescents with newly diagnosed acute lymphoblastic leukemia (ALL).
Patients and methods: Patients achieving complete remission (CR) on Dana-Farber Cancer Institute ALL Consortium Protocol 00-01 were eligible for random assignment to 1) dexamethasone or prednisone, administered as 5-day pulses, every 3 weeks, and 2) weekly EC-Asnase, administered as a 25,000 IU/m(2) fixed dose (FD) or individualized dose (ID) starting at 12,500-IU/m(2), adjusted every 3 weeks based on nadir serum asparaginase activity (NSAA) determinations.
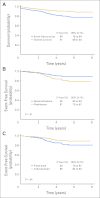
Results: Between 2000 and 2004, 492 evaluable patients (ages 1 to 18 years) enrolled; 473 patients (96%) achieved CR. Four hundred eight patients (86%) participated in the corticosteroid randomization and 384 patients (81%) in the EC-Asnase randomization. With 4.9 years of median follow-up, dexamethasone was associated with superior 5-year event-free survival (EFS; 90% v 81% for prednisone; P = .01) but higher rates of infection (P = .03) and, in older children, higher cumulative incidence of osteonecrosis (P = .02) and fracture (P = .06). ID EC-Asnase had superior 5-year EFS (90% v 82% for FD; P = .04), but did not reduce the frequency of asparaginase-related toxicity. Multivariable analysis identified both dexamethasone and ID EC-Asnase as independent predictors of favorable EFS.
Conclusion: There was no overall difference in skeletal toxicity by corticosteroid type; dexamethasone was associated with more infections and, in older children, increased incidence of osteonecrosis and fracture. There was no difference in asparaginase-related toxicity by EC-Asnase dosing method. Dexamethasone and ID EC-Asnase were each associated with superior EFS. Monitoring NSAA during treatment with EC-Asnase may be an effective strategy to improve outcome in pediatric ALL.
Trial registration: ClinicalTrials.gov NCT00165178.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Jones B, Freeman AI, Shuster JJ, et al. Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol. 1991;19:269–275. - PubMed
-
- Gaynon PS, Lustig RH. The use of glucocorticoids in acute lymphoblastic leukemia of childhood: Molecular, cellular, and clinical considerations. J Pediatr Hematol Oncol. 1995;17:1–12. - PubMed
-
- Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: A report from the Children's Cancer Group. Blood. 2003;101:3809–3817. - PubMed
-
- Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: Results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129:734–745. - PubMed
-
- Igarashi S, Manabe A, Ohara A, et al. No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children's Cancer Study Group L95-14 protocol. J Clin Oncol. 2005;23:6489–6498. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

