Role of ubiquitin ligases and the proteasome in oncogenesis: novel targets for anticancer therapies
- PMID: 23358974
- PMCID: PMC3807137
- DOI: 10.1200/JCO.2012.44.0958
Role of ubiquitin ligases and the proteasome in oncogenesis: novel targets for anticancer therapies
Abstract
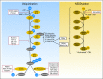
The ubiquitin proteasome system (UPS) regulates the ubiquitination, and thus degradation and turnover, of many proteins vital to cellular regulation and function. The UPS comprises a sequential series of enzymatic processes using four key enzyme families: E1 (ubiquitin-activating enzymes), E2 (ubiquitin-carrier proteins), E3 (ubiquitin-protein ligases), and E4 (ubiquitin chain assembly factors). Because the UPS is a crucial regulator of the cell cycle, and abnormal cell-cycle control can lead to oncogenesis, aberrancies within the UPS pathway can result in a malignant cellular phenotype and thus has become an attractive target for novel anticancer agents. This article will provide an overall review of the mechanics of the UPS, describe aberrancies leading to cancer, and give an overview of current drug therapies selectively targeting the UPS.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Wilkinson KD. Ubiquitin: A Nobel protein. Cell. 2004;119:741–745. - PubMed
-
- Reinstein E, Ciechanover A. Narrative review: Protein degradation and human diseases—The ubiquitin connection. Ann Intern Med. 2006;145:676–684. - PubMed
-
- Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005;12:1191–1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

