Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial
- PMID: 23359039
- PMCID: PMC3589308
- DOI: 10.1503/cmaj.121265
Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial
Abstract
Background: Most patients with type 1 diabetes do not achieve their glycemic targets. We aimed to assess the efficacy of glucose-responsive insulin and glucagon closed-loop delivery for controlling glucose levels in adults with type 1 diabetes.
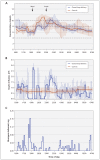
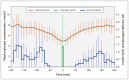
Methods: We conducted a randomized crossover trial involving 15 adults with type 1 diabetes, comparing standard insulin-pump therapy with dual-hormone, closed-loop delivery. Patients were admitted twice to a clinical research facility and received, in random order, both treatments. Each 15-hour visit (from 1600 to 0700) included an evening exercise session, followed by a medium-sized meal, a bedtime snack and an overnight stay. During visits that involved closed-loop delivery, basal insulin and glucagon miniboluses were delivered according to recommendations based on glucose sensor readings and a predictive dosing algorithm at 10-minute intervals. During visits involving standard insulin-pump therapy (control visits), patients used conventional treatment.
Results: Dual-hormone closed-loop delivery increased the percentage of time for which patients' plasma glucose levels were in the target range (median 70.7% [interquartile range (IQR) 46.1%-88.4%] for closed-loop delivery v. 57.3% [IQR 25.2%-71.8%] for control, p = 0.003) and decreased the percentage of time for which plasma glucose levels were in the low range (bottom of target range [< 4.0 mmol/L], 0.0% [IQR 0.0%-3.0%] for closed-loop delivery v. 10.2% [IQR 0.0%-13.0%] for control, p = 0.01; hypoglycemia threshold [< 3.3 mmol/L], 0.0% [IQR 0.0%-0.0%] for closed-loop delivery v. 2.8% [IQR 0.0%-5.9%] for control, p = 0.006). Eight participants (53%) had at least 1 hypoglycemic event (plasma glucose < 3.0 mmol/L) during standard treatment, compared with just 1 participant (7%) during closed-loop treatment (p = 0.02).
Interpretation: Dual-hormone, closed-loop delivery guided by advanced algorithms improved short-term glucose control and reduced the risk of hypoglycemia in a group of 15 adults with type 1 diabetes.
Trial registration: ClinicalTrials.gov, no. NCT01297946.
Figures
Comment in
-
The future of care for type 1 diabetes.CMAJ. 2013 Mar 5;185(4):285-6. doi: 10.1503/cmaj.130011. Epub 2013 Jan 28. CMAJ. 2013. PMID: 23359043 Free PMC article. No abstract available.
References
-
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group N Engl J Med 1993;329:977–86 - PubMed
-
- Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–67 - PMC - PubMed
-
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002; 45:937–48 - PubMed
-
- Hypoglycemia in the Diabetes Control and Complications Trial; The Diabetes Control and Complications Trial Research Group Diabetes 1997;46:271–86 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
