Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors
- PMID: 23359047
- PMCID: PMC3589328
- DOI: 10.1503/cmaj.120744
Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors
Abstract
Background: Clinical trials are commonly done without blinded outcome assessors despite the risk of bias. We wanted to evaluate the effect of nonblinded outcome assessment on estimated effects in randomized clinical trials with outcomes that involved subjective measurement scales.
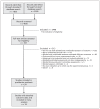
Methods: We conducted a systematic review of randomized clinical trials with both blinded and nonblinded assessment of the same measurement scale outcome. We searched PubMed, EMBASE, PsycINFO, CINAHL, Cochrane Central Register of Controlled Trials, HighWire Press and Google Scholar for relevant studies. Two investigators agreed on the inclusion of trials and the outcome scale. For each trial, we calculated the difference in effect size (i.e., standardized mean difference between nonblinded and blinded assessments). A difference in effect size of less than 0 suggested that nonblinded assessors generated more optimistic estimates of effect. We pooled the differences in effect size using inverse variance random-effects meta-analysis and used metaregression to identify potential reasons for variation.
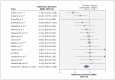
Results: We included 24 trials in our review. The main meta-analysis included 16 trials (involving 2854 patients) with subjective outcomes. The estimated treatment effect was more beneficial when based on nonblinded assessors (pooled difference in effect size -0.23 [95% confidence interval (CI) -0.40 to -0.06]). In relative terms, nonblinded assessors exaggerated the pooled effect size by 68% (95% CI 14% to 230%). Heterogeneity was moderate (I(2) = 46%, p = 0.02) and unexplained by metaregression.
Interpretation: We provide empirical evidence for observer bias in randomized clinical trials with subjective measurement scale outcomes. A failure to blind assessors of outcomes in such trials results in a high risk of substantial bias.
Figures

References
-
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Oxford (UK): The Cochrane Collaboration; 2011
-
- Haahr MT, Hróbjartsson A. Who is blind in randomised clinical trials? An analysis of 200 trials and a survey of authors. Clin Trials 2006;3:360–5 - PubMed
-
- Poolman RW, Struijs PA, Krips R, et al. Reporting of outcomes in orthopaedic randomized trials: Does blinding of outcome assessors matter? J Bone Joint Surg Am 2007;89:550–8 - PubMed
-
- Liu CJ, LaValley M, Latham NK. Do unblinded assessors bias muscle strength outcomes in randomized controlled trials of progressive resistance strength training in older adults? Am J Phys Med Rehabil 2011;90:190–6 - PubMed
-
- Pildal J, Hróbjartsson A, Jørgensen KJ, et al. Impact of allocation concealment on conclusions drawn from meta-analyses of randomised trials. Int J Epidemiol 2007;36:847–57 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
