TGFβ-induced PI 3 kinase-dependent Mnk-1 activation is necessary for Ser-209 phosphorylation of eIF4E and mesangial cell hypertrophy
- PMID: 23359369
- PMCID: PMC3855027
- DOI: 10.1002/jcp.24327
TGFβ-induced PI 3 kinase-dependent Mnk-1 activation is necessary for Ser-209 phosphorylation of eIF4E and mesangial cell hypertrophy
Abstract
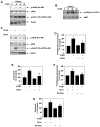
Transforming growth factorβ (TGFβ)-induced canonical signal transduction is involved in glomerular mesangial cell hypertrophy; however, the role played by the noncanonical TGFβ signaling remains largely unexplored. TGFβ time-dependently stimulated eIF4E phosphorylation at Ser-209 concomitant with enhanced phosphorylation of Erk1/2 (extracellular signal regulated kinase1/2) and MEK (mitogen-activated and extracellular signal-regulated kinase kinase) in mesangial cells. Inhibition of Erk1/2 by MEK inhibitor or by expression of dominant negative Erk2 blocked eIF4E phosphorylation, resulting in attenuation of TGFβ-induced protein synthesis and mesangial cell hypertrophy. Expression of constitutively active (CA) MEK was sufficient to induce protein synthesis and hypertrophy similar to those induced by TGFβ. Pharmacological or dominant negative inhibition of phosphatidylinositol (PI) 3 kinase decreased MEK/Erk1/2 phosphorylation leading to suppression of eIF4E phosphorylation. Inducible phosphorylation of eIF4E at Ser-209 is mediated by Mnk-1 (mitogen-activated protein kinase signal-integrating kinase-1). Both PI 3 kinase and Erk1/2 promoted phosphorylation of Mnk-1 in response to TGFβ. Dominant negative Mnk-1 significantly inhibited TGFβ-stimulated protein synthesis and hypertrophy. Interestingly, inhibition of mTORC1 activity, which blocks dissociation of eIF4E-4EBP-1 complex, decreased TGFβ-stimulated phosphorylation of eIF4E without any effect on Mnk-1 phosphorylation. Furthermore, mutant eIF4E S209D, which mimics phosphorylated eIF4E, promoted protein synthesis and hypertrophy similar to TGFβ. These results were confirmed using phosphorylation deficient mutant of eIF4E. Together our results highlight a significant role of dissociation of 4EBP-1-eIF4E complex for Mnk-1-mediated phosphorylation of eIF4E. Moreover, we conclude that TGFβ-induced noncanonical signaling circuit involving PI 3 kinase-dependent Mnk-1-mediated phosphorylation of eIF4E at Ser-209 is required to facilitate mesangial cell hypertrophy.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures



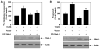



Similar articles
-
Raptor-rictor axis in TGFbeta-induced protein synthesis.Cell Signal. 2008 Feb;20(2):409-23. doi: 10.1016/j.cellsig.2007.10.027. Epub 2007 Nov 7. Cell Signal. 2008. PMID: 18068336
-
TGFβ acts through PDGFRβ to activate mTORC1 via the Akt/PRAS40 axis and causes glomerular mesangial cell hypertrophy and matrix protein expression.J Biol Chem. 2020 Oct 16;295(42):14262-14278. doi: 10.1074/jbc.RA120.014994. Epub 2020 Jul 30. J Biol Chem. 2020. PMID: 32732288 Free PMC article.
-
TGFβ-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion.PLoS One. 2012;7(8):e42316. doi: 10.1371/journal.pone.0042316. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22879939 Free PMC article.
-
Targeting Mnks for cancer therapy.Oncotarget. 2012 Feb;3(2):118-31. doi: 10.18632/oncotarget.453. Oncotarget. 2012. PMID: 22392765 Free PMC article. Review.
-
A patent review of mitogen-activated protein kinase-interacting kinases (MNKs) modulators (2019-present).Expert Opin Ther Pat. 2024 Dec 26:1-14. doi: 10.1080/13543776.2024.2446225. Online ahead of print. Expert Opin Ther Pat. 2024. PMID: 39708134 Review.
Cited by
-
Inhibition of Mitogen-activated Protein Kinase (MAPK)-interacting Kinase (MNK) Preferentially Affects Translation of mRNAs Containing Both a 5'-Terminal Cap and Hairpin.J Biol Chem. 2016 Feb 12;291(7):3455-67. doi: 10.1074/jbc.M115.694190. Epub 2015 Dec 14. J Biol Chem. 2016. PMID: 26668315 Free PMC article.
-
TGFβ-induced deptor suppression recruits mTORC1 and not mTORC2 to enhance collagen I (α2) gene expression.PLoS One. 2014 Oct 15;9(10):e109608. doi: 10.1371/journal.pone.0109608. eCollection 2014. PLoS One. 2014. PMID: 25333702 Free PMC article.
-
Ethanol acutely antagonizes the refeeding-induced increase in mTOR-dependent protein synthesis and decrease in autophagy in skeletal muscle.Mol Cell Biochem. 2019 Jun;456(1-2):41-51. doi: 10.1007/s11010-018-3488-4. Epub 2018 Dec 6. Mol Cell Biochem. 2019. PMID: 30523512 Free PMC article.
-
High glucose enhances microRNA-26a to activate mTORC1 for mesangial cell hypertrophy and matrix protein expression.Cell Signal. 2015 Jul;27(7):1276-85. doi: 10.1016/j.cellsig.2015.03.007. Epub 2015 Mar 20. Cell Signal. 2015. PMID: 25797045 Free PMC article.
-
Deeping in the Role of the MAP-Kinases Interacting Kinases (MNKs) in Cancer.Int J Mol Sci. 2020 Apr 23;21(8):2967. doi: 10.3390/ijms21082967. Int J Mol Sci. 2020. PMID: 32340135 Free PMC article. Review.
References
-
- Abboud HE. Mesangial cell biology. Exp Cell Res. 2012;318:979–985. - PubMed
-
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. - PubMed
-
- Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. - PubMed
-
- Das F, Ghosh-Choudhury N, Mahimainathan L, Venkatesan B, Feliers D, Riley DJ, Kasinath BS, Choudhury GG. Raptor-rictor axis in TGFbeta-induced protein synthesis. Cell Signal. 2008a;20:409–423. - PubMed
-
- Das F, Ghosh-Choudhury N, Venkatesan B, Li X, Mahimainathan L, Choudhury GG. Akt kinase targets association of CBP with SMAD 3 to regulate TGFbeta-induced expression of plasminogen activator inhibitor-1. J Cell Physiol. 2008b;214:513–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

