An intact brachyury function is necessary to prevent spurious axial development in Xenopus laevis
- PMID: 23359630
- PMCID: PMC3554630
- DOI: 10.1371/journal.pone.0054777
An intact brachyury function is necessary to prevent spurious axial development in Xenopus laevis
Abstract
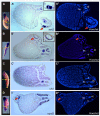
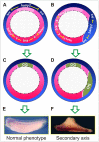
We have previously shown that the member of the HES family hairy2 induces the ectopic expression of dorsal markers when it is overexpressed in the ventral side of Xenopus embryos. Intriguingly, hairy2 represses the mesoderm transcription factor brachyury (bra) throughout its domain in the marginal zone. Here we show that in early gastrula, bra and hairy2 are expressed in complementary domains. Overexpression of bra repressed hairy2. Interference of bra function with a dominant-negative construct expanded the hairy2 domain and, like hairy2 overexpression, promoted ectopic expression of dorsal axial markers in the ventral side and induced secondary axes without head and notochord. Hairy2 depletion rescued the ectopic dorsal development induced by interference of bra function. We concluded that an intact bra function is necessary to exclude hairy2 expression from the non-organiser field, to impede the ectopic specification of dorsal axial fates and the appearance of incomplete secondary axes. This evidence supports a previously unrecognised role for bra in maintaining the dorsal fates inhibited in the ventral marginal zone, preventing the appearance of trunk duplications.
Conflict of interest statement
Figures


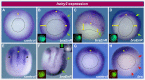
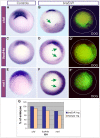


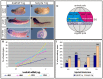
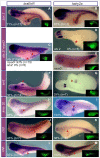



References
-
- Dobrovolskaïa-Zavadskaïa N (1927) Sur la mortification spontanée de la queue chez la souris nouveau-née et sur l’existence d’un caractère heriditaire “nonviable”. C R Soc Biol 97: 114–116.
-
- Chesley P (1935) Development of the short-tailed mutant in the house mouse. J Exp Zool 70: 429–459.
-
- Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H (1990) Cloning of the T gene required in mesoderm formation in the mouse. Nature 343: 617–622. - PubMed
-
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG (1991) Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67: 79–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

