Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing
- PMID: 23359649
- PMCID: PMC3556677
- DOI: 10.1038/srep01150
Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing
Abstract
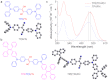
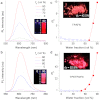
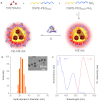
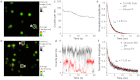
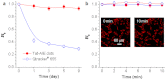
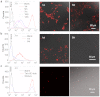
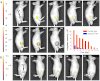
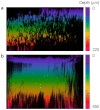
Long-term noninvasive cell tracing by fluorescent probes is of great importance to life science and biomedical engineering. For example, understanding genesis, development, invasion and metastasis of cancerous cells and monitoring tissue regeneration after stem cell transplantation require continual tracing of the biological processes by cytocompatible fluorescent probes over a long period of time. In this work, we successfully developed organic far-red/near-infrared dots with aggregation-induced emission (AIE dots) and demonstrated their utilities as long-term cell trackers. The high emission efficiency, large absorptivity, excellent biocompatibility, and strong photobleaching resistance of the AIE dots functionalized by cell penetrating peptides derived from transactivator of transcription proteins ensured outstanding long-term noninvasive in vitro and in vivo cell tracing. The organic AIE dots outperform their counterparts of inorganic quantum dots, opening a new avenue in the development of fluorescent probes for following biological processes such as carcinogenesis.
Figures
References
-
- Ly H. Q., Frangioni J. V. & Hajjar R. J. Imaging in cardiac cell-based therapy: in vivo tracking of the biological fate of therapeutic cells. Nat. Clin. Pract. Cardiovasc. Med. 5, S96–S102 (2008). - PubMed
-
- Schächinger V. et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the TOPCARE-AMI Trial. J. Am. Coll. Cardiol. 44, 1690–1699 (2004). - PubMed
-
- Ebert S. N. et al. Noninvasive tracking of cardiac embryonic stem cells in vivo using magnetic resonance imaging techniques. Stem Cells 25, 2936–2944 (2007). - PubMed
-
- Doubrovin M. M. et al. In vivo imaging and quantitation of adoptively transferred human antigen-specific T cells transduced to express a human norepinephrine transporter gene.Cancer Res. 67, 11959–11969 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

